About Teleflex Inc
Teleflex Incorporated (Teleflex) is a global provider of medical technology products that enhance clinical benefits, improve patient and provider safety and reduce total procedural costs.
The company primarily designs, develops, manufactures and supplies single-use medical devices used by hospitals and healthcare providers for common diagnostic and therapeutic procedures in critical care and surgical applications. The company markets and sells its products to hospitals and healthcare providers worldwide through a combination of its direct sales force and distributors. The company’s products are used in numerous markets and for a variety of procedures.
Segments
The company operates through four segments: Americas, EMEA (Europe, the Middle East and Africa), Asia (Asia Pacific) and OEM (Original Equipment Manufacturer and Development Services).
Each of the company’s three geographic segments provides a comprehensive portfolio of medical technology products used by hospitals and healthcare providers. However, certain of the company’s products are more heavily concentrated within certain segments. For example, most of the company’s urology products are sold by its EMEA segment and most of its interventional urology products are sold by its Americas segment.
The company’s OEM segment designs, manufactures and supplies devices and instruments for other medical device manufacturers. The company’s OEM division, which includes the TFX Medical OEM, TFX OEM, Deknatel and HPC Medical brands, provides custom extrusions, micro-diameter film-cast tubing, diagnostic and interventional catheters, balloons and balloon catheters, film-insulated fine wire, coated mandrel wire, conductors, sheath/dilator introducers, specialized sutures and performance fibers, bioabsorbable sutures, yarns and resins.

Products
The company’s product categories within its geographic segments include vascular access, anesthesia, interventional, surgical, interventional urology, respiratory and urology. Each of these categories and the key products sold therein are described in more detail below
.
Vascular Access: The company’s Vascular Access product category offers devices that facilitate a variety of critical care therapies and other applications with a focus on helping reduce vascular-related complications. These products primarily consist of the company’s Arrow branded catheters, catheter navigation and tip positioning systems and its intraosseous, or in the bone, access systems.
The company’s catheters are used in a wide range of procedures, including the administration of intravenous therapies, the measurement of blood pressure and the withdrawal of blood samples through a single puncture site. Many of the company’s catheters provide antimicrobial and antithrombogenic protection technology that has been shown to reduce the risk of catheter related bloodstream infections and microbial colonization and thrombus accumulation on catheter surfaces.
The company’s intraosseous access systems are designed for the delivery of medications and fluids when intravenous access is difficult to obtain in emergent, urgent or medically necessary cases. The company’s products offer a method for vascular access that can be administered quickly and effectively in the hospital and pre-hospital environments and include the EZ-IO Intraosseous Vascular Access System and Arrow FAST1 Sternal Intraosseous Infusion System.
Interventional: The company’s Interventional product category offers devices that facilitate a variety of applications to diagnose and deliver treatment of coronary and peripheral vascular disease. These products primarily consist of a variety of coronary catheters, structural heart support devices, peripheral intervention products and mechanical circulatory support platform used by interventional cardiologists, interventional radiologists and vascular surgeons. Clinical benefits of the company’s products include increased vein and artery access, post-procedure closure, and increased support during complex medical procedures. The company’s primary product offerings consist of a portfolio of Arrow branded intra-aortic balloon pumps and catheters, GuideLiner, Turnpike and TrapLiner catheters, the MANTA Vascular Closure device and Arrow OnControl powered bone biopsy system.
Anesthesia: The company’s Anesthesia product category consists of airway, pain management and hemostatic product lines that support hospital, emergency medicine and military channels.
The company’s airway management products and related devices are designed to enable use of standard and advanced anesthesia techniques in both pre-hospital emergency and hospital settings. The company’s key products include laryngoscopes, supraglottic airways, endotracheal tubes and atomization devices, which are branded under its LMA, Rusch and MAD trade names.
The company’s pain management product line includes epidurals, catheters and disposable pain pumps for regional anesthesia, designed to improve patients’ post-operative pain experience, which are branded under its Arrow trade name.
The company’s hemostatic products accelerate the body's natural clotting cascade and are used in trauma situations where bleeding is difficult to control. The portfolio consists of external hemostats used by first responders, interventional products used in the catheter lab, and trauma products used by trauma surgeons, which are branded under its QuikClot trade name.
Surgical: The company’s Surgical product category consists of single-use and reusable devices designed for use in a variety of surgical procedures. These products primarily consist of metal and polymer ligating clips, fascial closure surgical systems used in laparoscopic surgical procedures, percutaneous surgical systems, a powered bariatric stapler, and other surgical instruments used in Ear, Nose and Throat and Cardio-Vascular and Thoracic procedures. The company’s significant surgical brands include Weck, MiniLap, Pleur-Evac, Deknatel, KMedic, Pilling and Titan SGS.
Interventional Urology: The company’s Interventional Urology product category includes the UroLift System, a minimally invasive technology for treating lower urinary tract symptoms due to benign prostatic hyperplasia, or BPH. The UroLift System involves the placement of permanent implants, typically through a transurethral outpatient procedure, that hold the prostate lobes apart to relieve compression on the urethra without cutting, heating or removing prostate tissue. In 2023, the company expanded its product portfolio with the acquisition of Palette Life Sciences AB (Palette), which adds a portfolio of hyaluronic acid gel-based products primarily utilized in the treatment of urological diseases, including Barrigel, a rectal spacing product used in connection with radiation therapy treatment of prostate cancer. The company’s Interventional Urology product portfolio is most heavily weighted in its Americas segment.
Respiratory: The company’s respiratory products are used in a variety of care settings and primarily consist of humidification and oxygen therapy products. This product category previously included aerosol therapy, spirometry and ventilation management products, as well as certain other oxygen therapy products, all of which were included in the Respiratory business divestiture.
Urology: The company’s urology product portfolio provides bladder management for patients in the hospital and individuals in the home care markets. The product portfolio consists principally of a wide range of catheters (including Foley and intermittent), urine collectors, catheterization accessories and products for operative endourology, which are marketed under the Teleflex and Rusch brand names. The company’s urology product portfolio is most heavily weighted in its EMEA segment.
Markets
The company generally serves three end-markets: hospitals and healthcare providers, medical device manufacturers and home care. These markets are affected by a number of factors, including demographics, utilization and reimbursement patterns.
Sales and Marketing
The company’s product sales are made directly to hospitals, healthcare providers, distributors and to original equipment manufacturers of medical devices through its own sales forces, independent representatives and independent distributor networks.
Patents and Trademarks
The company owns a portfolio of patents, patents pending and trademarks. The company also licenses various patents and trademarks. Patents for individual products extend for varying periods based upon the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The Teleflex name and the Arrow and UroLift brands are essential to the operation of the company’s business.
Seasonality
Portions of the company’s revenues are subject to seasonal fluctuations. Incidence of flu and other disease patterns and, to a lesser extent, the frequency of elective medical procedures affect revenues related to single-use products. Historically, the company’s has experienced higher sales in the fourth quarter as a result of these factors.
Government Regulation
All of the company’s medical devices manufactured or distributed in the U.S. are subject to requirements set forth by the Federal Food, Drug, and Cosmetic Act (FDC Act) and regulations promulgated by the FDA under the FDC Act, which are enforced by the FDA. The FDA and, in some cases, other government agencies administer requirements for the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, servicing, marketing, importing and exporting of all finished devices intended for human use. Additional FDA requirements include premarket clearance and approval, advertising and promotion, distribution and post-market surveillance of the company’s medical devices and establishment of registration and device listing for its facilities.
Unless an exemption, pre-amendment grandfather status (that is, medical devices legally marketed in the U.S. before May 28, 1976) or FDA enforcement discretion applies, each medical device that the company markets in the U.S. must first receive either clearance as a Class I or, typically, a Class II device (after submitting a premarket notification (510(k)) or approval as a Class III device (after filing a premarket approval application (PMA)) from the FDA pursuant to the FDC Act. To obtain 510(k) clearance, a manufacturer must demonstrate to the FDA that the proposed device is substantially equivalent to a legally marketed device (a 510(k)-cleared device, a pre-amendment device for which FDA has not called for PMAs or a device with a de novo authorization), referred to as the predicate device. Substantial equivalence is established by the applicant showing that the proposed device has the same intended use as the predicate device, and it either has the same technological characteristics or has been shown to be equally safe and effective and does not raise different questions of safety and effectiveness as compared to the predicate device. The FDA’s 510(k) clearance process requires regulatory competence to execute and usually takes four to nine months, but it can last longer. A device that is not eligible for the 510(k) process because there is no predicate device may be reviewed by the FDA through the de novo process (the process for
The company’s portfolio of existing products and pipeline of potential new products consist primarily of Class I (510(k) exempt) and Class II devices that require 510(k) clearance, although a few are 510(k)-exempt. In addition, certain modifications made to devices after they receive clearance or approval may require a new 510(k) clearance or approval of a PMA or PMA supplement.
Certain of the company’s medical devices are sold in kits that include a drug component, such as lidocaine. These types of kits are generally regulated as combination products within the Center for Devices and Radiological Health (CDRH) under the device regulations because the device provides the primary mode of action of the kit. Although the kit as a whole is regulated as a medical device, it may be subject to certain drug requirements such as current good manufacturing practices (cGMPs) and adverse drug experience reporting requirements, to the extent applicable to the drug-component repackaging activities and subject to inspection to verify compliance with cGMPs as well as other regulatory requirements.
The company’s manufacturing facilities, as well as those of certain of its suppliers, are subject to periodic and for-cause inspections by FDA personnel to verify compliance with the QSR (21 CFR Part 820) as well as other regulatory requirements. Similar inspections and audits are performed by Notified Bodies to verify compliance to applicable ISO standards (e.g. ISO 13485:2016), by auditing organizations under the Medical Device Single Audit Program (MDSAP) applicable to regulatory requirements of Australia, Brazil, Canada, Japan and the U.S., and/or by regulatory authorities to verify compliance with medical device regulations and requirements from the countries in which it distributes product.
The company is also subject to the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws applicable in jurisdictions outside the U.S. that generally prohibit companies and their intermediaries from improperly offering or paying anything of value to non-U.S. government officials for the purpose of obtaining or retaining business. In the sale, delivery and servicing of the company’s medical devices and software outside of the U.S., it must also comply with various export control and trade embargo laws and regulations, including those administered by the Department of Treasury’s Office of Foreign Assets Control (OFAC) and the Department of Commerce’s Bureau of Industry and Security (BIS) which may require licenses or other authorizations for transactions relating to certain countries and/or with certain individuals identified by the U.S. government.
History
Teleflex Incorporated was founded in 1943. The company, a Delaware corporation, was incorporated in 1943.

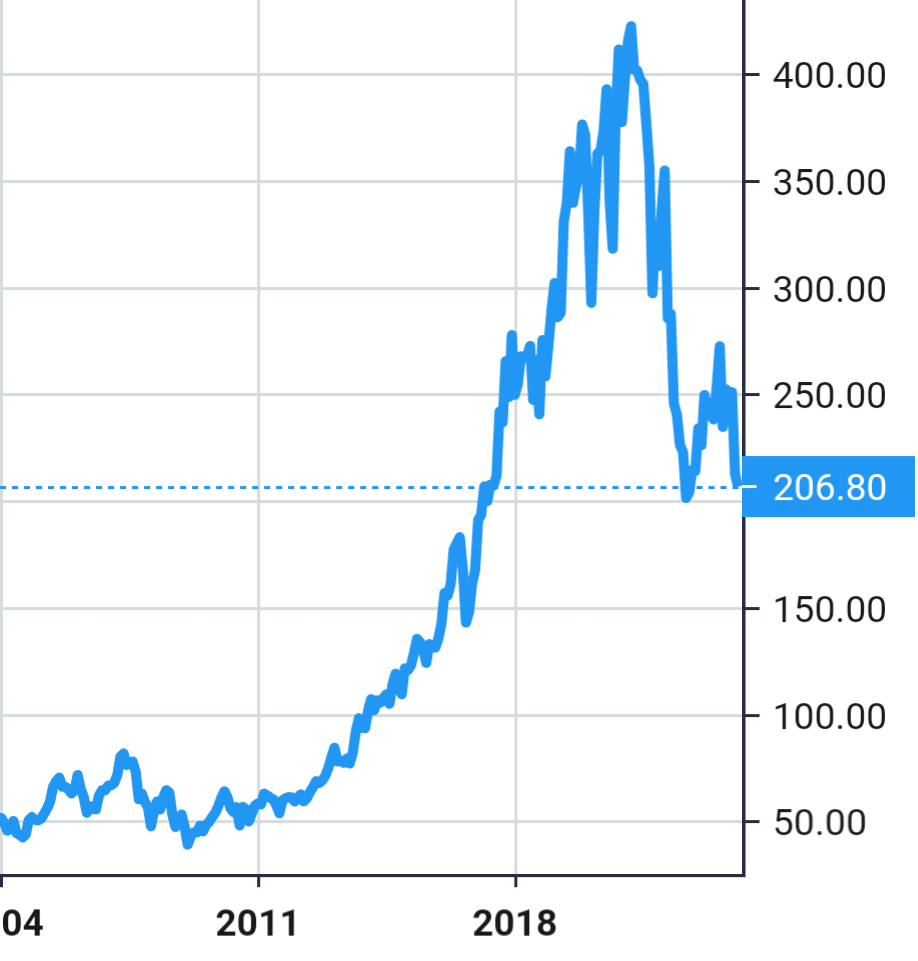
 Stock Value
Stock Value

