About PerkinElmer
Revvity, Inc. (Revvity) provides health science solutions, technologies, expertise and services that deliver complete workflows from discovery to development, and diagnosis to cure.
Revvity is revolutionizing what’s possible in healthcare, with specialized focus areas in translational multi-omics technologies, biomarker identification, imaging, prediction, screening, detection and diagnosis, informatics and more. The company markets its products and services in more than 160 countries.
The company’s customers include pharmaceutical and biotechnology companies, laboratories, academic and research institutions, public health authorities, private healthcare organizations, doctors and government agencies. Many of the company’s products are used by its customers to develop, test, and manufacture their products.
The company ships a significant portion of its products to its customers through independent package delivery and import/export companies, including UPS and Federal Express in the United States; TNT, UPS and DHL in Europe; and UPS in Asia. The company also ships its products through other carriers, including commercial airlines, freight carriers, national trucking firms, overnight carrier services and the United States Postal Service.
Strategy
The company’s strategy is to develop and deliver innovative products, services and solutions in high-growth markets that utilize the company’s knowledge and expertise to address customers’ critical needs and drive scientific breakthroughs. To execute on the company’s strategy and accelerate revenue growth, the company focuses on broadening its offerings through both the investment in research and development and the acquisition of innovative technology.

The company’s strategy includes strengthening the company’s position within key markets by expanding the company’s global product and service offerings, maintaining superior product quality and driving an enhanced customer experience; accelerating transformational innovation through both internal research and development and third-party collaborations and alliances; augmenting growth in both of the company’s core business segments, Life Sciences and Diagnostics, through strategic acquisitions and licensing; engraining focused operational excellence to improve organizational efficiency and agility; and opportunistically utilizing the company’s share repurchase programs to help drive shareholder value.
Dispositions
On March 13, 2023, the company completed the sale of certain assets and the equity interests of certain entities constituting the company’s Applied, Food and Enterprise Services businesses to PerkinElmer Topco, L.P., a Delaware limited partnership owned by funds managed by affiliates of New Mountain Capital L.L.C.
Business Segments and Products
The company reports its business in two segments: Life Sciences and Diagnostics.
Life Sciences segment
The company’s comprehensive portfolio of technologies helps life sciences researchers better understand diseases and develop treatments. The company provides a broad suite of products, solutions and services that facilitate optimized workflows, increase productivity, and accelerate every stage of the drug discovery and development pipeline. The company’s offerings span the areas of cell, gene, and protein research, enabling scientists to work smarter, make research breakthroughs, and transform those breakthroughs into real-world outcomes. The company partners with global pharmaceutical, biotech and contract research organizations, as well as academic institutions, to enable them to discover and develop better treatments and therapeutics to fight disease faster and more efficiently.
Principal Products
The company’s principal products and services for Life Sciences applications include the following:
Radiometric detection solutions, including over 750 radiochemicals and instrumentation such as the Tri-Carb and Quantulus GCT families of liquid scintillation analyzers, Wizard2 Gamma counters and MicroBeta2 plate based LSA, which are used for beta, gamma and luminescence counting in microplate and vial formats utilized in research, environmental and drug discovery applications.
The Opera Phenix Plus high-content screening system, which is used for sensitive and high-speed phenotypic drug screening of complex cellular models.
The Operetta CLS high-content analysis system, which enables scientists to reveal fine sub-cellular details from everyday assays as well as more complex studies, for example using live cells, 3D and stem cells.
Reagents and solutions for microscopy and imaging applications. These include PhenoVue cellular imaging reagents and cell painting kits, PhenoPlate (formerly CellCarrier Ultra) cellular imaging microplates and GrowDex hydrogels, fluorophore-conjugated and enzyme-conjugated antibodies, as well as buffers and solutions, such as the company’s Ce3D collection of buffers for 3D tissue imaging.
The MuviCyte live-cell imaging system, designed to operate inside a cell-culture incubator, enabling researchers to study cellular behaviors and pathways in living cells to gain a deeper understanding of functions, disease mechanisms and responses to treatments.
The Signals Image Artist next-generation image analysis and management platform for drug discovery research, to help scientists process and analyze high-content screening (HCS) and cellular imaging data in a matter of hours versus days or weeks, so they can make more informed decisions faster.
The VICTOR Nivo multimode plate reader benchtop system, which is designed for assay development and academic labs, including those using HTRF and AlphaLISA assay technologies.
The EnSight multimode plate reader benchtop system, which offers well plate imaging alongside labeled detection technologies for target-based and phenotypic assays.
The EnVision multimode plate reader, which is designed for high-throughput screening laboratories, including those using HTRF, AlphaScreen and AlphaLISA assay technologies.
A wide range of homogeneous biochemical and cell-based reagents using HTRF, LANCE Ultra, DELFIA, AlphaLISA, AlphaLISA SureFire Ultra, AlphaScreen, AlphaPlex and luminescence assay technologies.
A broad portfolio of recombinant GPCR and ion channel cell lines, including over 300 products and 120 ready-to-use frozen cell lines for a wide range of disease areas.
BioLegend ELISA MAX Standard Sets, ELISA MAX Deluxe Sets, LEGEND MAX ELISA Kits and RAPID MAX ELISA Kits as well as complementary solutions and buffers for immunoassays to cover more than 200 targets for human, mouse, and rat samples, many of which are designed to assess the immune environment and its inflammatory state for vaccine, infectious disease and autoimmune disease research.
BioLegend LEGENDplex bead-based reagents, which, in contrast to single analyte assays such as enzyme-linked immunosorbent assays (‘ELISAs’), can quantitate up to 14 targets, from one small sample volume in a flow cytometry assay, and include both desktop and cloud-based analysis software.
In vivo imaging technologies and reagents for preclinical research, consisted of the IVIS Spectrum series for 2D and 3D optical imaging and optionally integrated low-dose CT imaging and the IVIS Lumina series for benchtop 2D imaging, along with IVISbrite bioluminescent and IVISense fluorescent imaging agents, cell lines and dyes.
The Quantum GX2 system, which enables low-dose in vivo CT imaging of multiple species and areas of anatomical interest across multiple disease areas by way of high-resolution, tomographic imaging.
GoInVivo as well as Ultra-LEAF and LEAF functional antibodies, which provide an affordable solution for researchers performing in vivo and ex vivo studies.
Nexcelom BioScience high-throughput, microwell Celigo image cytometry system, Cellaca MX high-throughput cell counter, the new Cellaca PLX image cytometry system, and Cellometer automated cell counters, complemented by consumables and reagents, including reagents and kits for cell counting assays and cell viability, microplates, slides, and counting beads.
Mimix Reference Standards, which are cell line-derived and suitable for Next Generation Sequencing, droplet-digital and Real-Time PCR as well as Sanger sequencing. The platform is agnostic for seamless integration into any quality control workflow.
Dharmacon Reagents and gene modulation technologies such as RNAi that support drug discovery and development for greater understanding of gene function, identify genetic drivers behind human disease, develop and validate diagnostic workflows, and help deliver biotherapeutics, cellular and gene therapies for precision medicine with a portfolio of cell engineering tools.
Pin-point base editing platform, which is a CRISPR-Cas9-based technology that allows researchers to make precision base changes in genomic DNA. Editing with such precision can be used to silence disease-causing genes, correct disease-associated mutations, and optimize cell therapies.
CHOSOURCE platform, which was expanded to include CHO-K1 ADCC+ expression cell line for the development of therapeutic antibodies in oncology, infectious disease and autoimmune conditions.
BioLegend’s catalog of more than 20,000 SKUs, incorporating antibodies and a large collection of antibody conjugates and modifications as well as recombinant proteins, immunoassays and other supportive reagents and solutions for cell and molecular analysis.
The T-SPOT Discovery SARS-CoV-2 research-use-only assay to investigate cell-mediated immunity related to COVID-19.
Sirion Biotech consultancy services and technologies to design and manufacture viral vectors for cell and gene therapy research and preclinical development.
BioLegend best-in-class antibodies, recombinant proteins and related reagents, which are used across multiple applications and research areas, including proteogenomics, tissue, cell and protein analysis, cancer research, immunology, cell and gene therapy, stem cell therapy and neuroscience.
Fluorophore-conjugated antibodies, which are used in flow cytometers to characterize protein expression on the surface and in internal compartments of cells. The large collection of dyes and antibodies allows for an increasing number of conjugate options, facilitating the use of bigger and better flow cytometry panels using conventional and spectral flow cytometers. Notable products are Brilliant Violet and the Spark and Fire dye series, among others.
BioLegend TotalSeq reagents, which are oligonucleotide-barcoded antibodies that enable protein detection by sequencing that can be combined with traditional RNA or DNA sequencing experiments with high-parameter protein detection, including comprehensive cloud-based analysis software.
Cell culture and biofunctional assay reagents, including bioactive recombinant proteins, as well as other specialized reagents such as Cell-Vive T-NK Xeno-Free Serum Substitute (compliant with Good Manufacturing Practice requirements (‘GMP’)), and other GMP-produced recombinant proteins and reagents. These products serve several markets, notably cell and gene therapy applications.
BioLegend’s MojoSort and Lymphopure reagents for cell separation that complement the company’s fluorophore-antibody conjugates, used for FACS (Fluorescence-activated Cell Sorting), thus covering most cell separation and cell sorting technologies and applications.
Flex-T reagents that utilize peptide-loaded major histocompatibility molecules assembled into tetramers for the identification of antigen-specific T cells. The company’s Flex-T products can be used to screen the efficacy of antigen peptides for vaccine and drug trials, as well as characterize the dominance of cancer-specific self-peptides, and more recently, SARS-CoV2 peptides for COVID-19 research.
Antibodies and solutions for Western blotting. A large collection of validated antibodies, as well as supporting buffers and substrates, which provide a convenient set of tools to characterize protein size and relative expression levels in cell or tissue lysates.
Signals Research Platform, which equips pharmaceutical scientists with the essential tools to gather, search, mine, analyze and visualize critical data, yielding actionable insights in an automated, predictive, and scalable manner. Within life science research and development and clinical research applications, the company’s software accelerates innovation, development, collaboration and research, ultimately leading to life-enhancing medical breakthroughs more quickly, promoting the company’s intention of a healthier humankind. In addition, it empowers scientists and formulators in specialty chemical and food sciences to analyze food, and additives, and create high-performing materials that align with sustainability initiatives, promoting energy efficiency, lower toxicity and a circular economy.
Signals Notebook, a secure cloud-native electronic lab notebook (ELN) for chemistry, biology, research, and formulations. From increased collaboration to securely accessible data, Signals Notebooks accelerates research and development workflows, increases collaboration, integrates with Microsoft Office and more.
Signals ChemDraw, which since 1985 has provided solutions with powerful capabilities and integrations to help quickly turn ideas and drawings into publications. Signals ChemDraw automates chemical drawings and transforms them into chemical knowledge by facilitating the management, reporting and presenting of chemistry research.
Signals Clinical, which provides a single unified platform to support data access, preparation and analytics, from source to visualization to action. With unrivaled workflow flexibility to support dynamic collaboration, Signals Clinical’s SaaS solution helps accelerate the delivery of urgently needed therapeutics to patients.
Vega preclinical ultrasound system, a hands-free, automated, high-throughput imaging system delivering high-resolution 2D and 3D ultrasound images in minutes. Originally launched in North America in 2022, it is now globally available.
New assay kits for Adeno-associated Virus Vectors (AAVs) and gene therapy applications in the company’s range of HTRF and AlphaLISA reagents, for detecting and quantifying CHO HCP impurities in biotherapeutics development, as well as kits across oncology, neuroscience, and targeted protein degradation applications.
Cellaca PLX image cytometry system, which combines best-in-class image cytometer hardware, software, validated consumables and optimized reagent kits with validated antibodies from the company’s BioLegend business, and trackable data reporting to enable the simultaneous detection of multiple markers and to streamline cell and gene therapy workflows.
New fluorescent stains, reagents and secondary antibodies in the company’s PhenoVue cellular imaging reagents portfolio for the detection and analysis of cellular components.
The latest version of the Signals Image Artist next-generation image analysis and management platform, which provides improved 3D cell segmentation and analysis, an AWS S3 cloud deployment option and enhanced cloud security, and compatibility with a broader range of systems, including the Nexcelom from Revvity Celigo image cytometer.
An updated VICTOR Nivo multimode plate reader with a new software version for streamlined data analysis.
OptiScint NPE-free scintillation cocktails and quench standards, providing a more environmentally friendly alternative without compromising performance.
Expansion of the company’s Western blotting reagents with the addition of the Western Lightning One range, which has a pre-mixed one component chemiluminescent HRP substrate for more consistent results.
Additional Spark and Fire dye-conjugated antibodies, enabling higher-parameter flow cytometry. Notable products are the Spark UV 387 and Spark Red 718 conjugates.
For the TotalSeq reagent portfolio, more large panels of pre-titrated oligo-conjugated antibodies released in Universal Panels for the analysis of human and mouse samples.
Software solutions for BioLegend LEGENDplex assays, multiomics analysis with TotalSeq reagents, and flow cytometry-based cell analysis software (Ryvett) that are now part of BioLegend’s data integration offerings.
New Products
New products introduced or acquired for Life Sciences applications in the year ended December 31, 2023 (fiscal year 2023) include the following:
Pin-point base editing reagents, which improve access to new-generation editing technology. The launch of these reagents puts clinically relevant base editing using the Pin-point platform in the hands of preclinical laboratories seeking to accelerate genomic insights and cell therapy research.
IVIS Spectrum 2 and IVIS SpectrumCT 2 next-generation imaging systems, the company’s newest flagship platforms setting the standard in high-throughput performance and versatility.
Quantum GX3 microCT imaging solution, a high-throughput system with superior spatial resolution and fast, low-dose scanning for diverse in vivo and biological ex vivo applications. With class-leading resolution, the system is designed for a wide applications, including bone imaging.
Signals Research Suite, a unified, cloud-native SaaS platform that drives scientific collaboration across research and development disciplines from drug discovery to specialty chemicals material development.
Signals DLX powered by Scitara,, which establishes seamless, bidirectional connectivity across instruments, LIMS, ELNs and other critical lab systems that previously existed in isolation.
Brand Names
The company’s Life Sciences segment offers additional products under various brand names:
Accell, AdenoBOOST, AlphaLISA, AlphaPlex, AlphaScreen, Alpha SureFire, Brilliant Violet, Ce3D, CellCarrier, Cellaca, Celigo, Cellometer, cell::explorer, Cell-Vive, Chalice, Chem3D, ChemDraw, ChemOffice, CHOSOURCE, Dharmacon, DharmaFECT, Edit-R, ELISA MAX, EnSight, EnVision, Flex-T, FMT, FolateRSense, GoInVivo, HTRF, IVIS, IVISbrite, IVISense, LANCE, LANCE Ultra , LEAF, LEGEND MAX, LEGENDplex, LentiBOOST, Lincode, Living Image, Lumina, Lymphopure, MicroBeta2, Mini ELISA Plate Reader, miRIDIAN, MojoSort, MuviCyte, ON-TARGET, ON-TARGETplus, Opera Phenix Plus, Operetta CLS, OptiScint, PhenoPlate, PhenoVue, Pin-point, Quantulus GCT, RAPID MAX, RediJect, RNAiONE, Signals, Signals Image Artist, SMARTpools, SMARTvector, Spark, Spectrum, TotalSeq, Tri-Carb, T-SPOT, Ultra-LEAF, Vega, VesselVue, ViaStain, VICTOR Nivo Western Lightning and Wizard2.
Diagnostics segment
The company offers instruments, reagents, assay platforms and software to hospitals, medical labs, clinicians and medical research professionals to help improve the health of families. The company’s Diagnostics segment is especially focused on reproductive health, immunodiagnostics, emerging market diagnostics and applied genomics.
The company provides early detection for genetic disorders from pregnancy to early childhood, and infectious disease testing for the diagnostics market. The company’s screening products are designed to provide early and accurate insights into the health of expectant mothers during pregnancy and into the health of their babies. Diagnostic labs use the company’s instruments, reagents and software for testing and screening genetic abnormalities and certain disorders and diseases, including Down syndrome, hypothyroidism, muscular dystrophy, infertility and various metabolic conditions. The company also develops technologies that enable and support genomic workflows using PCR and next-generation DNA sequencing for applications in oncology, immunodiagnostics and drug discovery.
Principal Products
The company’s principal products and services for Diagnostics applications include the following:
The DELFIA Xpress screening platform, a complete solution for prenatal and maternal health screening, which includes a fast continuous loading system. It is supported by kits for first, second and third trimester analyses for prenatal screening and clinically validated LifeCycle software.
The DELFIA Xpress sFlt-1 kit, which enables short term prediction of pre-eclampsia and aids in diagnosis in the second and third trimesters of pregnancy together with the previously launched DELFIA Xpress PlGF 1-2-3 assay.
The NeoBase non-derivatized MS/MS AAAC kits, which are used to support detection of metabolic disorders in newborns through tandem mass spectrometry. The kits analyze newborn dry blood spot samples for measurement of amino acids and other metabolic analytes for specific diseases.
The GSP Neonatal hTSH, T4 17á-OHP, GALT IRT, BTD, PKU, Total Galactose, CK-MM and G6PD kits, used for screening congenital neonatal conditions from a drop of blood.
The Specimen Gate informatics data management solution, designed specifically for newborn screening laboratories.
The NeoLSD MS/MS kit, the first commercial IVD kit for screening of Pompe, MPS-I, Fabry, Gaucher, Niemann-Pick A/B and Krabbe disorders from a single dried blood spot sample.
QSight 210MD and 225MD UHPLC MS/MS instruments, which are used for newborn screening.
ViaCord umbilical cord blood banking services for the banking of stem cells harvested from umbilical cord blood and cord tissue, for potential therapeutic application in transplant and regenerative medicine.
An expanded portfolio of molecular-based infectious disease screening technologies for blood bank and clinical laboratory settings in China. The tools include a qualitative 3-in-1 assay for the detection of hepatitis B, hepatitis C and HIV, as well as assays for other communicable diseases.
TRF-based Anti HBs/HCV/TP kits for infectious disease testing.
Chitas instrument and HBV/HCV/HIV 3-in-1 PCR reagents for blood screening, and Hi Sensitivity HBV DNA and HCV RNA assays for clinical infectious disease testing.
The Bead Ruptor Elite Bead Mill Homogenizer, which enables grinding, lysing, and homogenizing of biological samples prior to molecular extraction delivering repeatable sample disassociation.
OMNI Prep 96 Automated Homogenizer Workstation, which is a fully automated homogenization workstation, enabling true walk-away processing.
The chemagic Prime instrument, a fully automated, LIMS-compatible solution for primary sample transfer, DNA and RNA isolation, optional normalization and the setup of PCR and Next Generation Sequencing (‘NGS’) applications.
Automated liquid handling platforms (Fontus, JANUS, Sciclone, Zephyr and FlexDrop) that offer a choice of robotic solutions in genomics, biotherapeutics, high throughput screening and high content analysis to assist life science research from bench to clinic.
JANUS BioTx and PreNAT II workstations for automated small-scale purification, offering column, tip and plate-based chromatography on a single platform.
HIVE CLX scRNAseq Solution, which integrates sample storage and single cell profiling into a complete workflow, solving the issues that limit single cell RNA analysis. The LabChip GXII Touch protein characterization system, which provides a means of characterizing multiple protein product attributes for research labs through QC.
The explorer automated workstation, which allows integration of multiple laboratory instrumentation using a centralized robotic interface, allowing high throughput and turnkey-application focused solutions.
Vanadis NIPT, a non-PCR non-sequencing fully automated cfDNA technology for use in any laboratory for screening common trisomies in the pregnant population.
Revvity Omics, a global laboratory network offering multi-OMIC clinical grade services for testing in cytogenetics, biochemical genetics (prenatal and postnatal), molecular genetics and immunodiagnostics. The laboratory network includes testing laboratories in the United States, Sweden, India, China and the United Kingdom.
The EONIS assay, a CE marked and the United States Food and Drug Administration (‘FDA’) authorized system utilizing real-time PCR technology, which allows for simultaneous screening of SMA, SCID and XLA in newborns from a single DBS punch.
Chemiluminescence immunoassays covering endocrinology, autoimmunity, infectious diseases, allergy and therapeutic drug monitoring.
ELISAs covering endocrinology, autoimmunity, diabetes monitoring, steroids, thyroid monitoring, animal research and tumor markers.
Radioactive immunoassays in calcium metabolism.
Autoimmune testing, including indirect immunofluorescence tests (IIFT), ELISA, chemiluminescence immunoassays and immunoblots, covering rheumatology, hepatology, gastroenterology, endocrinology, neurology, nephrology, dermatology and infertility.
Allergy testing covering allergen-specific immunoglobin E (IgE), measuring the level of different IgE antibodies or total IgE in blood using EUROLINE immunoblot assays.
Infectious disease testing, including IIFT, ELISA, chemiluminescence immunoassays, immunoblots, microarrays and real-time PCR, covering bacteria, viruses, fungi and parasites.
A complete portfolio of chemiluminescence immunoassays (‘ChLIA’) for precise Alzheimer’s disease diagnostics, which provides reliable analysis of the established CSF biomarkers beta-amyloid (1-40), beta-amyloid (1-42), total tau and pTau (181) and a high degree of standardization due to fully automated processing.
EUROLabPolaris, which provides the secure transfer of indirect immunofluorescence data to several locations enabling central evaluation within the software.
Laboratory management system EUROLabOffice 4.0, which provides a central interface between devices to simplify and speed up the diagnostic routine and increases security through organization of all lab procedures and traceable documentation of all data and processes.
EUROLabWorkstation IFA and EUROLabWorkstation ELISA, which provide fully automated processing of IIFT and ELISA, respectively, for laboratories with high sample throughput.
EUROPattern Microscope, which provides fully automated immunofluorescence microscopy including IIFT pattern recognition and titer determination.
EUROPattern Microscope live, which provides fully automated and fast image recording and modern on-screen reporting, also including IIFT pattern recognition and titer determination.
EUROBlotOne, a compact tabletop device for complete processing of immunoblots.
IDS-i10, a compact random access solution for the processing of ChLIA in the field of autoimmune and infection diagnostics as well as antigen detection, providing sample throughput of up to 60 samples per hour.
IDS-iSYS Multi-Discipline Automated System, which is a compact automation solution for the processing of ChLIA in the field of autoimmune, infection and allergy diagnostics, as well as antigen detection, providing sample throughput of up to 120 samples per hour.
Pre-NAT II, which provides fully automated high-throughput sample preparation for molecular genetic diagnostics, consisting of nucleic acid extraction and subsequent pipetting of the PCRs.
MyFoodProfile immunoblots for the determination of IgG and IgE reactivity against more than 200 foods (CE-marked).
Prenatal and postnatal testing utilizing Revvity Omics Next Generation Sequencing products, including gene panels, exomes and genomes.
Revvity Omics Whole Genome Sequencing test, which detects duo genome (nuclear and mitochondrial) single nucleotide and copy number variants and includes screening for pharmacogenetic variants, Spinal Muscular Atrophy and more than 30 short tandem repeat disorders.
Revvity Omics test for Facioscapularhumeral dystrophy (FSHD) using Genome Optical Mapping technology.
Oxford Immunotec T-SPOT Technology platform, a modified ELISPOT used to detect a T cell immune response to infection.
Oxford Immunotec T-SPOT.TB test, an in vitro diagnostic test for the detection of effector T cells that respond to stimulation by mycobacterium tuberculosis antigens by capturing interferon gamma in the vicinity of T cells in human whole blood. It is intended for use as an aid in the diagnosis of tuberculosis infection.
The PG-Seq Rapid Kit v2, which analyzes picogram quantities of DNA from an embryo biopsy for preimplantation genetic research with enhanced whole genome coverage and accuracy.
DOPlify WGA V2 Kit, which performs fast whole genome amplification on single cells or limited template DNA samples, allowing cell chromosome copy number status to be determined.
chemagic 360-D instrument (IVDR) and chemagic Prime Junior-D instrument (IVDR) for automated nucleic acid isolation, and related kits, such as CE-IVD chemagic Nucleic Acid Purification Kits and chemagic miRNA 200 Kit.
Revvity Omics WholePanel test, which is an enhanced panel testing (WholeCancer, WholeAtaxia, WholeCardiology and WholeMuscularDystrophy panels) using genome sequencing as a backbone and provides full intronic coverage and short tandem repeat screening in one test.
Revvity Omics UltraRapid Whole Genome Sequencing with StepOne, CMV detection and metagenomic analysis, which provides the sickest babies in NICUs with multiomic testing results in five days or less.
New Products
New products or services introduced or acquired for Diagnostics applications in fiscal year 2023 include the following:
Fontus liquid handler, which is available in multiple versions to automate both NGS and life science workflows.
EONIS Q, a novel ‘dry-chemistry’ qPCR newborn screening workflow for SCID and SMA screening.
DELFIA Trio, an automated plate dispenser, washer, disk remover for the manual newborn screening and prenatal workflows.
EVOYA, a cloud-based, newborn screening, informatics and data management software.
Automation protocols and kits launched for the BioQule NGS System, making it an open system that combines automation, reagents, consumables and scripts, enabling walkaway automation to simplify low throughput NGS library preparation and quantitation.
UNIQO160, a device for fully automated processing of IIFT from primary sample to final microscopy result for up to 160 samples and 18 slides.
89 new IIFT for the ultrafast automated microscope ‘EUROPattern Microscope Live’.
Anti-TBE Virus ELISA 2.0 (IgG) and Anti-TBE Virus CSF ELISA 2.0 (IgG) for detection of IgG antibodies against TBE virus in serum and CSF, respectively (CE-marked, IVDR-compliant).
Brand Names
The company’s Diagnostics segment offers additional products under various brand names, including AutoDELFIA, BACS-on-Beads, BIOCHIPs, Bioo Scientific, BoBs, chemagic, Chitas, DELFIA, DELFIA Xpress, DOPlify, EONIS, EUROArray , EUROIMMUN, EUROLabWorkstation, EUROLINE, EUROPatternTM, Evolution Evoya, explorer, Fontus, Genoglyphix, GSP, Haoyuan, IDS Immunodiagnosticsystems, IDS-i10, IDS-i10T, IDS-iSYS, iLab, iQ, JANUS, LabChip, LifeCycle, LimsLink, Migele, MultiPROBE, NEXTFLEX, NextPrep, Pannoramic, Panthera Puncher, PG-Seq, PG-Find PKamp, PreNAT II, Prime, Protein Clear, ProteinEXact, QSight, QuantiVac, RONIA, Sciclone, SimplicityChrom, Specimen Gate, Superflex, Symbio, T-SPOT, Touch, Twister, Vanadis, VariSpec, ViaCord, VICTOR 2 D, and Zephyr.
Marketing
All of the company’s businesses market their products and services primarily through their own specialized sales forces. As of December 31, 2023, the company employed approximately 1,400 sales and service representatives operating in approximately 40 countries and marketing products and services in more than 160 countries. In geographic regions where the company does not have a sales and service presence, the company utilizes distributors to sell its products.
Regulatory Affairs
The company’s operations are subject to regulation by different state and federal government agencies in the United States and other countries, as well as to the standards established by international standards bodies. Some of the company’s products are subject to regulation by the FDA and similar foreign agencies.
The company is also subject to a variety of laws, regulations and standards that govern, among other things, the importation and exportation of products; and the company’s business practices in the United States and abroad, such as anti-bribery, anti-corruption and competition laws.
Research and Development Expenses
The company’s research and development expenses were $216.6 million for the year ended December 31, 2023.
History
The company, a Massachusetts corporation, was founded in 1937. It was incorporated in 1947. The company was formerly known as PerkinElmer, Inc. and changed its name to Revvity, Inc. in 2023.

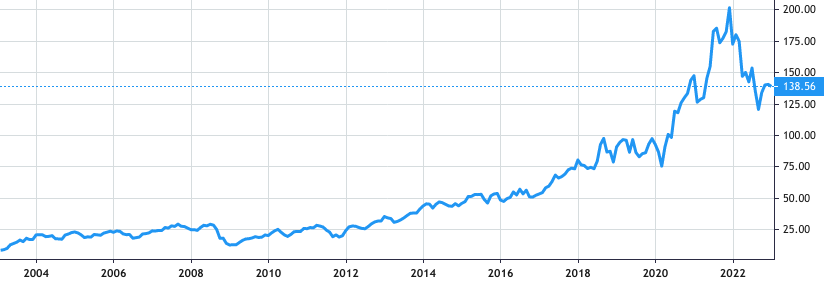
 Stock Value
Stock Value

