About Edwards Lifesciences Corp
Edwards Lifesciences Corporation operates as a manufacturer of heart valve systems and repair products used to replace or repair a patient's diseased or defective heart valve worldwide. The company is the global leader in patient-focused medical innovations for structural heart disease and critical care monitoring.
The company’s innovative work in heart valves encompasses both surgical and transcatheter therapies for heart valve replacement and repair. In addition, the company’s robust pipeline of future technologies focuses on the less invasive repair or replacement of the mitral and tricuspid valves of the heart, which are more complex and more challenging to treat than the aortic valve. The company is also a global leader in hemodynamic and noninvasive brain and tissue oxygenation monitoring systems used to measure a patient's cardiovascular function in the hospital setting.
Cardiovascular disease is the number-one cause of death in the world and is the top disease in terms of health care spending in nearly every country. In the U.S. alone, one cardiovascular patient dies every 33 seconds. Cardiovascular disease is progressive in that it tends to worsen over time and often affects the structure of an individual's heart.
Patients undergoing treatment for cardiovascular disease can be treated with a number of the company’s medical technologies, which are designed to address individual patient needs with respect to disease process, comorbidities, and health status.
Edwards Lifesciences' Product and Technology Offerings
The company’s products and technologies are categorized into four main groups: Transcatheter Aortic Valve Replacement, Transcatheter Mitral and Tricuspid Therapies, Surgical Structural Heart, and Critical Care.

Transcatheter Aortic Valve Replacement
The company is the global leader in transcatheter heart valve replacement technologies designed for the minimally-invasive replacement of aortic heart valves. The Edwards SAPIEN family of valves, including the Edwards SAPIEN 3, the Edwards SAPIEN 3 Ultra, and the Edwards SAPIEN 3 Ultra RESILIA systems, are catheter-based approaches for treating patients who have severe symptomatic aortic stenosis. The SAPIEN 3 valves are delivered while the heart is still beating. The majority of procedures are conducted without the use of general anesthesia and patients are discharged home within one to two days. Transcatheter aortic valve replacement with the SAPIEN 3 family of valves enables patients to recover more quickly and return to a better quality of life sooner than patients receiving traditional open heart surgical therapies. Edwards' transcatheter aortic heart valves were first commercialized in Europe in 2007, in the United States in 2011, and in Japan in 2013. Edwards has partnered with the physician community to generate groundbreaking data that has expanded access to patients of all risk profiles. In 2023, The PARTNER 3 Trial, which demonstrated a 99% freedom from stroke or mortality at 1 year, had 5-year data presented with a 90% freedom from all-cause mortality. The SAPIEN 3 platform remains the only transcatheter heart valve with a THV-in-THV indication for patients assessed at high-risk for surgical replacement, offering patients the ability to have a second minimally invasive procedure. The SAPIEN family of valves are the most widely implanted transcatheter heart valves in the world with over one million patients lives impacted since launch. Additionally, the Edwards SAPIEN 3 system and Alterra system offer a minimally invasive option for pulmonary valve replacement for patients with congenital heart disease.
Sales of the company’s transcatheter aortic valve replacement products represented 65% of its net sales in 2023.
Transcatheter Mitral and Tricuspid Therapies
The company continues to make significant investments in the development of transcatheter heart valve repair and replacement technologies designed to treat mitral and tricuspid valve diseases. While many of these technologies are in development and clinical phases, the PASCAL PRECISION and Cardioband transcatheter valve repair systems are commercially available in Europe for mitral and tricuspid valve repair. As of 2023, the PASCAL PRECISION system is also commercially available in the U.S. and Japan for degenerative mitral regurgitation patients. The PASCAL PRECISION system addresses the needs of patients with mitral or tricuspid regurgitation through leaflet approximation, while the Cardioband system enables clinicians to reduce the valve's annulus and lower regurgitation. In addition to transcatheter repair, transcatheter replacement is key to unlocking the full mitral and tricuspid opportunity. The company’s mitral replacement strategy positions it for leadership in the mid-to-long term. SAPIEN M3 is based on the proven SAPIEN valve and is designed specifically for mitral patients. For tricuspid valve replacement, the company’s EVOQUE system is delivered through a low-profile transfemoral sub 30-French system, and available in a variety of valve sizes to enable treatment in a wide range of patient anatomies. In 2023, the EVOQUE system received CE Mark approval for the transcatheter treatment of eligible patients with tricuspid regurgitation. More recently, in February 2024, the EVOQUE system received United States Food and Drug Administration (FDA) approval for the treatment of tricuspid regurgitation. This development will give a wide range of U.S. patients access to a treatment option that not only has the potential to improve quality-of-life, but also showed favorable clinical trends in all-cause mortality, re-intervention, and heart failure hospitalizations. The EVOQUE system is the world's first transcatheter valve replacement therapy to receive regulatory approval to treat tricuspid regurgitation.
Surgical Structural Heart
The company continues to invest in bringing innovations to cardiac surgery patients. The company’s RESILIA tissue, with published clinical data showing 99% freedom from structural valve deterioration through seven years1, has set the new standard for tissue valve durability. The company’s flagship INSPIRIS RESILIA aortic valve, offers RESILIA tissue and VFit technology. INSPIRIS is the leading aortic surgical valve in the world. Sales of the company’s surgical therapies in the United States also continue to gain traction with KONECT RESILIA, the first pre-assembled, ready to implant, tissue valved conduit for complex combined procedures.
The company’s latest innovation, the MITRIS RESILIA valve, is commercially available in Europe, as well as other geographies, including the U.S. and Japan, where it has been strongly adopted by surgeons as the leading product in its mitral valve portfolio. The demand for surgical structural heart therapies is growing worldwide, and that the company’s innovation strategy will continue to strengthen its leadership and positive impact on patients.
Sales of the company’s surgical tissue heart valve products represented 16% of its net sales in 2023.
Critical Care
The company is the world leader in advanced hemodynamic monitoring systems used to measure a patient's heart function and fluid status in surgical and intensive care settings. Edwards’ complete hemodynamic portfolio helps clinicians make proactive clinical decisions that can improve patient recovery. The portfolio includes the minimally-invasive FloTrac and Acumen IQ sensors, the noninvasive ClearSight and Acumen IQ cuffs, and the ForeSight noninvasive tissue oximetry sensor. The company also supports clinical needs with its well-established Swan-Ganz pulmonary artery catheters and arterial pressure monitoring products. Compatible with the company’s portfolio of sensors and catheters, the HemoSphere monitoring platform displays valuable physiological information. The company’s first predictive algorithm, Acumen Hypotension Prediction Index software, alerts clinicians in advance of a patient developing dangerously low blood pressure. The company’s latest algorithm, Acumen Assisted Fluid Management software, provides patient-specific fluid suggestions to help keep patients in an optimal range during surgery.
On December 7, 2023, the company announced plans for a tax-free spin-off of its Critical Care product group as a separate publicly traded company to Edwards Lifesciences' shareholders. Critical Care is integrating a full range of smart monitoring technologies into the seventh generation of its HemoSphere platform, creating a unique offering of enhanced recovery tools.
Competition
In Transcatheter Aortic Valve Replacement, the company’s primary competitors include Medtronic PLC, Abbott Laboratories (Abbott), and Boston Scientific Corporation. In Transcatheter Mitral and Tricuspid Therapies, the company’s primary competitor is Abbott, and there are a considerable number of large and small companies with development efforts in these fields. In Surgical Structural Heart, the company’s primary competitors include Medtronic PLC, Abbott, and Artivion, Inc. In Critical Care, the company competes primarily with a variety of companies in specific product lines including ICU Medical, Inc., PULSION Medical Systems SE, a subsidiary of Getinge AB, Cheetah Medical, Inc., a subsidiary of Baxter International, and LiDCO Group PLC, a subsidiary of Masimo Corporation.
Sales and Marketing
The company’s portfolio includes some of the most recognizable cardiovascular device product brands in treating structural heart disease today. The company has a number of product lines that require sales and marketing strategies tailored to deliver high-quality, cost-effective products and technologies to customers worldwide. Because of the diverse global needs of the population that the company serves, its distribution system consists of several direct sales forces, as well as independent distributors.
To achieve optimal outcomes for patients, the company conducts educational symposia and best practices training for its physician, hospital executive, service line leadership, nursing, and clinical-based customers. The company relies extensively on its sales and field clinical specialist personnel who work closely with its customers in hospitals. Field clinical specialists routinely attend procedures where Edwards' products are being used in order to provide guidance on the use of the company’s devices, thereby enabling physicians and staff to reach expert proficiency and deliver positive patient outcomes. In addition to working closely with physicians, nurses, and other clinical personnel, its customers include decision makers such as service line leaders, material managers, biomedical staff, hospital administrators and executives, purchasing managers, and ministries of health. Also, for certain of its product lines and where appropriate, the company’s corporate sales team actively pursues approval of Edwards Lifesciences as a qualified supplier for hospital group purchasing organizations (GPOs) that negotiate contracts with suppliers of medical products. Additionally, the company has contracts with a number of United States and European national and regional buying groups, including healthcare systems and Integrated Delivery Networks. Where it chooses to market its products is also influenced by the existence of, or potential for, adequate reimbursement to hospitals and other providers by national healthcare systems.
United States: In the United States, the company sells substantially all of its products through its direct sales forces. In 2023, 58% of the company’s net sales were derived from sales to customers in the United States.
Outside of the United States: In 2023, 42% of the company’s net sales were derived outside of the United States through its direct sales forces and independent distributors. Of the total sales outside of the United States, 53% were in Europe, 18% were in Japan, and 28% were in Rest of World. The company sells its products in approximately 100 countries, including Japan, Germany, France, the United Kingdom, Italy, China, and Canada. A majority of the sales and marketing approach outside of the United States is direct sales, although it varies depending on each country's size and state of development.
Government Regulation and Other Matters
The company’s products and facilities are subject to regulation by numerous government agencies, including the FDA, European Union member states competent authorities, and the Japanese Pharmaceuticals and Medical Devices Agency, to confirm compliance with the various laws and regulations governing the development, testing, manufacturing, labeling, marketing, and distribution of its products. The company is also subject to additional laws and regulations that govern its business operations, products, and technologies, including federal, state, and foreign anti-kickback laws and regulations, which generally prohibit payments to anyone, including physicians as an inducement to purchase or recommend a product; the Stark law, which prohibits physicians from referring Medicare or Medicaid patients to a provider that bills these programs for the provision of certain designated health services if the physician (or a member of the physician's immediate family) has a financial relationship with that provider; federal and state laws and regulations that protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of such information, in particular, the Health Insurance Portability and Accountability Act of 1996; the Physician Payments Sunshine Act, which requires public disclosure of the financial relationships of United States physicians and teaching hospitals with applicable manufacturers, including medical device, pharmaceutical, and biologics companies; the False Claims Act, which prohibits the submission of false or otherwise improper claims for payment to a federally funded health care program, and health care fraud statutes that prohibit false statements and improper claims to any third-party payor; and the United States Foreign Corrupt Practices Act, which can be used to prosecute United States companies for arrangements with foreign government officials or other parties, or for not keeping accurate financial records or maintaining adequate internal controls to prevent and detect arrangements with foreign government officials or other parties.
In Europe, the company’s products are subject to extensive regulatory requirements. The delivery of the company’s products is subject to regulation by the United States Department of Health and Human Services (HHS) and comparable state and foreign agencies responsible for reimbursement and regulation of health care items and services.
Seasonality
The company’s quarterly sales are influenced by many factors, including new product introductions, acquisitions, regulatory approvals, patient and physician holiday schedules, and other factors. Sales in the third quarter (year ended December 31, 2023) are typically lower than other quarters of the year due to the seasonality of the United States and European markets, where summer vacation schedules normally result in fewer medical procedures.
History
Edwards Lifesciences Corporation was founded in 1958. The company was incorporated in Delaware in 1999.

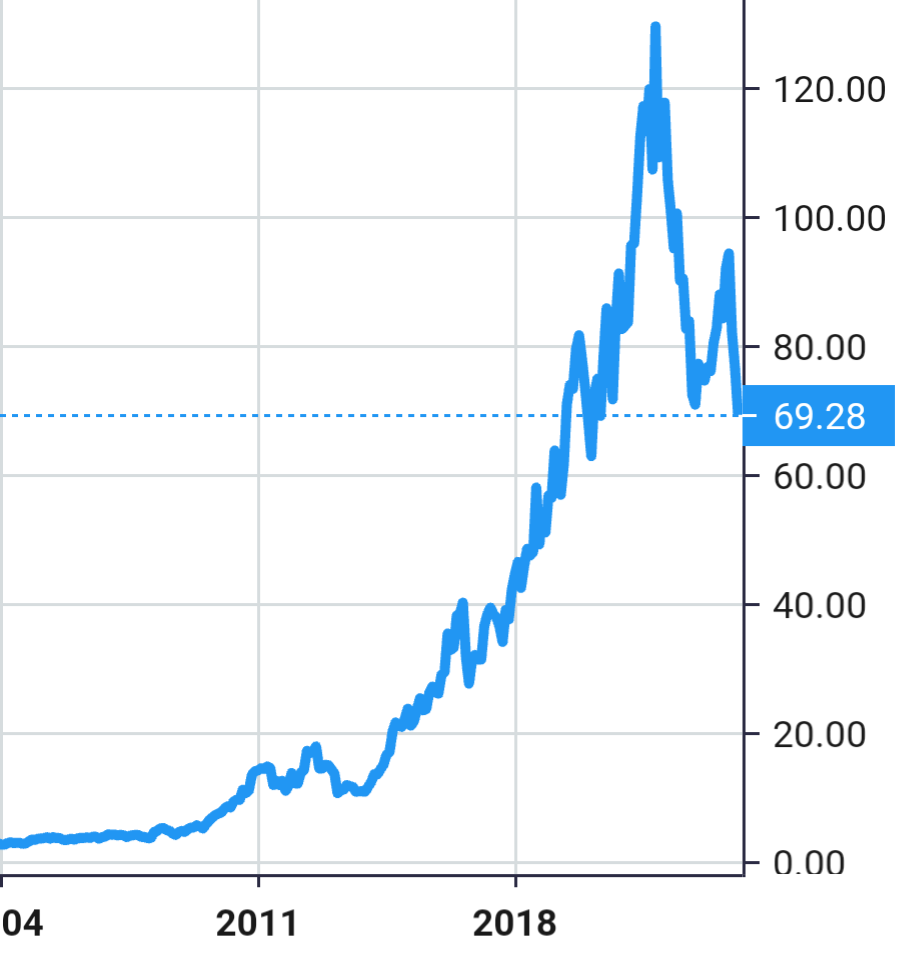
 Stock Value
Stock Value

