About Merck KGaA
MERCK Kommanditgesellschaft auf Aktien operates as a science and technology company worldwide.
The company provides a range of pharmaceutical and chemical products for various industries. The company has three business sectors: Healthcare, Life Science, and Performance Materials. These include the company’s six businesses.
Healthcare business sector
The company’s Healthcare business sector comprises the four businesses: Biopharma, Consumer Health, Biosimilars, and Allergopharma.
Biopharma
The company’s Biopharma business discovers, develops, manufactures, and markets pharmaceutical and biological prescription drugs to treat cancer, multiple sclerosis (MS), infertility and growth disorders, as well as certain cardiovascular and metabolic diseases. The company offers major brands in specialty medicine indications. The company is advancing its research and development (R&D) portfolio across the areas of oncology, immuno-oncology and immunology; and continues to invest in developing programs in MS. With the company’s expertise in discovery and early development, as well as approximately 25 projects in clinical development, it is focused on delivering differentiated new therapies to patients with unmet medical needs.

Biopharma’s primary medicine is Rebif (interferon beta-1a), an important product for people living with MS. Erbitux is the second primary drug in the portfolio of the Biopharma business and its major product in oncology. The product is a standard of care in multiple lines of metastatic colorectal cancer (mCRC) therapy, as well as of both recurrent/metastatic and locally advanced squamous cell carcinoma of the head & neck (SCCHN).
In 2014, the company entered into a global strategic alliance with Pfizer Inc. to develop and commercialize avelumab, an investigational anti-PD-L1 antibody initially discovered and developed by the company and in co-development as a potential treatment for multiple tumor types. The alliance is designed to boost the two companies’ presence in immuno-oncology. Both companies have also agreed to combine resources and expertise to advance Pfizer’s preclinical-stage anti-PD-1 antibody (PF-06801591) into Phase I trials. In 2015, together with Pfizer, the company initiated six pivotal trials for avelumab, including firstand second-line non-small cell lung cancer (NSLC), platinum-resistant ovarian cancer, first- and third-line gastric cancer, and first-line bladder cancer. Additionally, avelumab is being investigated in a Phase II study of patients with metastatic Merkel cell carcinoma.
As part of the strategic alliance, the company is co-promoting Pfizer’s anaplastic lymphoma kinase (ALK) inhibitor Xalkori (crizotinib), a medicine to treat ALK+ metastatic non-small cell lung cancer, in the United States and various other key markets. Under the agreement, Xalkori is being co-promoted in two waves, the first of which started in the second and third quarters of 2015 in the United States, Canada, Japan and five European Union countries (France, Germany, Italy, Spain, and the United Kingdom). In the United States and Canada, Xalkori is being co-promoted by EMD Serono, the brand under which the company’s U.S. and Canadian Biopharma business operates. The second wave would begin in 2016 and includes China and Turkey.
The co-promotion term would last through December 31, 2020 for Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States. It would run from January 1, 2016 through December 31, 2021 in China and Turkey.
In December 2015, the company announced its decision not to pursue evofosfamide (hypoxia-activated prodrug) further in soft tissue sarcoma and pancreatic cancer since, despite signs of activity in locally advanced and metastatic pancreatic cancer, two Phase III studies did not meet pre-specified primary endpoints. The company therefore decided not to pursue the evofosfamide development program further.
The company’s Biopharma business also offers products that help couples to conceive a child. To build on its strengths in fertility hormones, the company is offering an additional portfolio of fertility technologies from incubation to freezing. This comprises the Gavi, Geri and Gems product lines. Gavi is the automated vitrification instrument, using an automated and standardized laboratory protocol. Geri is a benchtop incubator with individually controlled incubation chambers per patient to minimize disruptive events to the early-stage embryo. Gems is the new generation of Genea Biomedx culture media allowing for embryo cultivation. Gavi and Geri received the CE mark clearance in Europe in 2015. The three product lines have not yet been cleared for use in the United States.
To further strengthen the company’s offering, its Biopharma business established the joint development hub ARTinnovations together with Genea. Founded to develop a pipeline of fertility technologies and services, ARTinnovations helps to support patients undergoing assisted reproductive technology (ART) and provides healthcare professionals with innovations to generate objective information to make important treatment decisions. Furthermore, the company formed the Global Fertility Alliance, a collaboration with Illumina Inc. and Genea Limited to advance excellence and standardization in Fertility. Also in 2015, the company launched a new version of the Eeva Test with the Xtend Algorithm, the advanced version of a non-invasive test to aid embryo assessment within assisted reproductive technology. The new version builds on the scientific and clinical record of the company’s Eeva System.
The General Medicine franchise mainly includes brands to treat cardiometabolic diseases. The main products of the Endocrinology franchise are Saizen (somatropin) and Kuvan (sapropterin dihydrochloride).
In October 2015, the company announced that it would return the rights for Kuvan to BioMarin to focus on its core businesses while giving patients continued support from a partner dedicated to orphan diseases. The company remains committed to patients in the field of endocrinology, and in particular to advancing the treatment of growth hormone-deficient patients with Saizen. Examples are the easypod electromechanical injection devices, the only growth hormone injection device of its kind, for the delivery of Saizen, and RebiSmart for Rebif (interferon beta-1a). Additionally, both easypod and RebiSmart are able to wirelessly transfer data, such as injection times, dates and doses to the Web-based software systems easypod connect and MSdialog.
Consumer Health
In the company’s Consumer Health business, it manufactures and markets over-the-counter pharmaceuticals and food supplements, focusing on various strategic brands. These include Neurobion, Bion, Seven Seas, Nasivin, Femibion, and Dolo- Neurobion, as well as Floratil, Sangobion, Vigantoletten, Apaisyl, and Kytta. The company has a high market penetration in Europe, Latin America, the Asia-Pacific, and Middle East and Africa.
In 2015, the company began the launch of its Bion brand in Brazil to add another major brand to the local portfolio. In addition, the Vigantol, Anemidox/Confer and Hepabionta brands were transferred from Biopharma to Consumer Health to leverage them through consumerization.
Biosimilars
The company’s Biosimilars business is committed to providing access to biologics to more patients worldwide. In addition, the company is developing a biosimilars portfolio focused on oncology and inflammatory disorders through both in-house research and development expertise in biologics and partnerships with other biosimilar players. In 2015, the company moved biosimilar candidates into clinical development. The first Phase III study for a biosimilar would be initiated in the first quarter of 2016.
The company has also established a strategic alliance with Dr. Reddy’s in India to co-develop multiple cancer drugs and with Bionovis in Brazil to supply the Brazilian market with biological products under the Product Development Partnership (PDP) policy of the Brazilian Ministry of Health.
Allergopharma
The company’s allergy business Allergopharma is a major company in the field of allergen immunotherapy (AIT). The Allergopharma portfolio includes a spectrum of approved allergen products that meet standards. The company manufactures products to diagnose and treat type 1 allergies, such as hay fever or allergic asthma. The company’s allergy business offers high-dose, hypoallergenic, standardized products for allergen immunotherapy of pollen and mite allergies. These allergoids have a special focus in Allergopharma’s product portfolio and constitute a cornerstone in its integrated health approach for patients suffering from these conditions. Allergopharma offers a range of diagnostics in the field of allergies with approximately 100 single allergens, providing physicians with the specific tools needed to identify the substances causing an allergy. In addition, Allergopharma provides individual allergen extracts on a named patient basis, which are needed to treat less frequent allergies. Products of Allergopharma are available in approximately 20 markets worldwide.
Life Science business sector
The purpose of the company’s Life Science business sector is to solve the life science problems by collaborating with the global scientific community. The company has a product and technology portfolio and offers solutions for scientists and engineers in the life science industry. The company’s products and services are used in the research, development and manufacture of biotechnological and pharmaceutical drug therapies, as well as in research and application laboratories. In addition, the company’s products and services reach adjacent markets, such as the food and beverage industry.
For the Life Science business sector, the most important event of 2015 was the completion in autumn 2015 of the acquisition of the Sigma-Aldrich Corporation (Sigma-Aldrich). The company’s Life Science business sector would be organized around three customer segments: Research Solutions focuses on academia, Process Solutions supports biopharmaceutical production, and Applied Solutions serves clinical and diagnostic testing laboratories, as well as the food and environmental industries.
The company’s new Magna ChIRP RNA Interactome Kits allow researchers to identify, recover and analyze regions of chromatin that interact with chromatin-associated RNAs, such as long non-coding RNA (lncRNA). The kits simplify the ChIRP method. The company’s Process Solutions business area offers a range of products to pharmaceutical and biotechnology companies that enable customers to develop large- and small-molecule drugs. In addition, the business area’s portfolio comprises approximately 400 chemicals for the synthesis of active pharmaceutical ingredients, as well as drug delivery compounds. The offering in biotech production comprises products supporting cell growth and gene expression, a range of filtration systems, and salts and sugars. In 2015, the company improved the application of its existing tangential flow filtration (TFF) technology that allows concentration of process streams without the recirculation required in traditional TFF.
A collaboration with the German company celares GmbH to provide PEGylation services to customers developing protein-based therapeutics and biosimilars was established. celares GmbH is a specialist for PEGylation, a special form of drug delivery for biopharmaceuticals. This collaboration enables the company to expand its service offering to include conjugation, further helping its biopharmaceutical and biosimilar customers to optimize their protein therapeutics and to reduce their time to market.
In addition, the company introduced improvements to its major EMPROVE portfolio of pharmaceutical raw materials in 2015. The expanded documentation and regulatory information facilitates drug product manufacturers’ risk assessment workflows and supplier qualification. The improvements also help drug product manufacturers meet their own internal quality guidelines, as well as those recently published by the European Commission.
Building on the company’s filtration portfolio, the company introduced Millipore Express PHF (process protection, high-flux) hydrophilic filters for fast, efficient and economical buffer filtration. A highlight of 2015 for Process Solutions was a strategic alliance with Turgut Ilaç, a major biosimilars company based in Turkey through which the business area would provide its Provantage End-to-End services for the development and manufacturing of biologics. Phase one of the agreement would focus on monoclonal antibody biosimilars for non-small cell lung carcinoma and rheumatoid arthritis, the first molecules of Turgut’s biosimilar pipeline that would be supported by the company under this strategic relationship.
Performance Materials
The company’s entire specialty chemicals business is consolidated in its Performance Materials business sector. The portfolio includes high-tech performance chemicals for applications in fields, such as consumer electronics, lighting, coatings, printing technology, paints, plastics, and cosmetics. Since January 1, 2015, Performance Materials has been organized into the following business units: Display Materials, Pigments & Functional Materials, Integrated Circuit Materials, and Advanced Technologies.
The company’s Liquid Crystals (LC) business, which is part of the Display Materials business unit, generated more than half of Performance Materials’ sales in 2015. Large LC display manufacturers are among the customers of the company’s Liquid Crystals business. It comprises the product offering for its customers in industry, including LC optimized for PS-VA (televisions) and IPS (smartphones and tablets) technologies. In addition, the company is continuously setting standards in new developments.
In 2015, the company focused on developing new application possibilities for LC, such as smart windows, so-called LC windows (LCWs). In 2014, the company acquired Peer+, a Dutch specialist for this technology; the company has meanwhile been fully integrated. In 2015, the first LCW panels were installed in the company’s new modular Innovation Center in Darmstadt.
The Pigments & Functional Materials business unit develops and markets a product portfolio of decorative effect pigments and functional materials. The effect pigments are primarily used in automotive and industrial coatings, plastics, printing applications, and cosmetics to give products a shine. Functional materials include laser marking, conductive additives, and applications for counterfeit protection, as well as cosmetic active ingredients, such as for use in skin care, sun protection and insect repellants.
The new Integrated Circuit Materials (ICM) business unit was established in 2015. ICM supplies products for integrated circuits. The products offered by ICM are used, among other things, to manufacture integrated circuits and microelectronic systems, for antireflection coatings, and for the miniaturization of transistor structures.
The Sigma-Aldrich SAFC Hitech business consisting of high-purity materials for silicon semiconductors, compound semiconductors and other high-tech applications is being integrated into the Integrated Circuit Materials business unit. It complements the company’s product offering as a major global supplier to the electronics and semiconductor industries. The company announced the acquisition of Ormet Circuits Inc. to further bolster the position of Integrated Circuit Materials as a manufacturer of semiconductor materials and to diversify the product portfolio. The Advanced Technologies business unit invests particularly in future-oriented research and development in Performance Materials. An example of this is the company’s materials for organic light-emitting diodes (OLEDs), which are used in new lighting techniques and display technologies.
In June, the company acquired the Israeli company, Qlight Nanotech, a major start-up for research in quantum materials which, among other things, could further improve the color properties of displays.

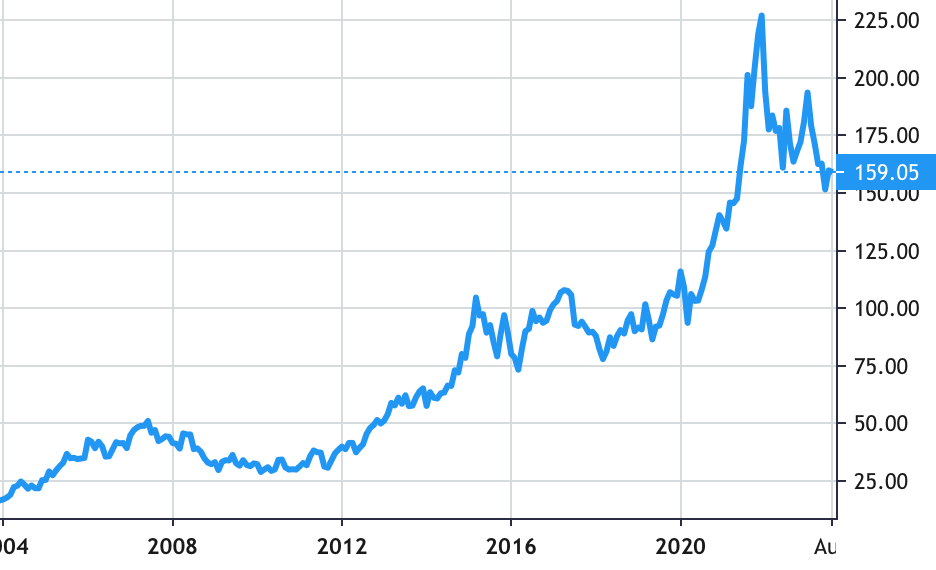
 Stock Value
Stock Value

