About Addex Therapeutics Ltd
Addex Therapeutics Ltd operates as a clinical-stage pharmaceutical company.
The company focuses on the development and commercialization of an emerging class of novel orally available small molecule drugs known as allosteric modulators.
As of December 31, 2022, the company's research and development efforts had been primarily focused on building a portfolio of proprietary drug candidates based on the company's allosteric modulator development capability. The allosteric modulator principle has broad applicability across a wide range of biological targets and therapeutic areas, but the company's primary focus is on G-protein coupled receptors, or GPCR, targets implicated in neurological diseases, where there is a clear medical need for new therapeutic approaches.
Using the company's allosteric modulator discovery capabilities, the company has developed a pipeline of proprietary clinical and preclinical stage drug candidates. The company or its partners are developing these clinical and preclinical stage proprietary drug candidates for diseases for which there are no approved therapies or where improved therapies are needed, including epilepsy, levodopa induced dyskinesia associated with Parkinson's disease, or PD-LID, substance use disorder, or, SUD, Charcot-Marie-Tooth type 1A neuropathy, or CMT1A, chronic cough, pain, stress related disorders, including post-traumatic stress disorder, or PTSD, schizophrenia and other neuropsychiatric and neurodegenerative disease. Some of these indications are classified as rare diseases, that may allow for orphan drug designation by regulatory agencies in major commercial markets, such as the United States, Europe and Japan. Orphan drug designation may entitle the recipient to benefits, in the jurisdiction granting the designation, such as market exclusivity following approval and assistance in clinical trial design, a reduction in user fees or tax credits related to development expenses.
The company's lead drug candidate, ADX71149 is a novel orally active metabotropic glutamate receptor subtype 2 positive allosteric modulator, or mGlu2 PAM for the treatment of epilepsy. The company's partner, Janssen Pharmaceuticals, Inc., or Janssen, a subsidiary of Johnson & Johnson is conducting a placebo-controlled Phase 2a proof of concept clinical trial of ADX71149 in epilepsy patients since June 2021, as well as an open label study since the third quarter of 2022. Part 1 of the study has been completed and an independent interim review committee, or IRC review to determine the next steps for the program is expected at the beginning of the second quarter of 2023. Under the company's agreement with Janssen, Janssen is responsible for financing the development and commercialization, if any, of ADX71149.
The company's second drug candidate, dipraglurant, a metabotropic glutamate receptor subtype 5 negative allosteric modulator, or mGlu5 NAM, is under evaluation for future development in a range of potential therapeutic indications, including PD-LID, stroke recovery, SUD and pain. As part of this evaluation, the company has initiated discussions with potential strategic partners with the objective of collaborating for future development. The company received orphan drug designation from the United States Food and Drug Administration, or FDA, for dipraglurant in PD-LID and completed a Phase 2 proof of concept study. On June 17, 2022, the company terminated its U.S. registration program, including a pivotal Phase 2B/3 study and an open label study in PD-LID due to slow recruitment of patients.

The company is also conducting a research program under the company's strategic partnership with Indivior PLC, or Indivior, to discover novel orally available gamma-aminobutyric acid subtype B receptor positive allosteric modulators, or GABAB PAMs. The company is in clinical candidate selection phase and expect IND enabling studies to be initiated in 2024. Under the terms of the agreement with Indivior, the company has the right to select drug candidates for development in certain exclusive indications outside SUD. The company plans to develop its selected drug candidates in CMT1a, chronic cough and pain, indications that have been clinically validated with baclofen, an orthosteric agonist of GABAB, and where there is a significant unmet medical need and commercial opportunity.
The company intends to continue to leverage its scientific expertise in allosteric modulation and the company's proprietary technology platform to discover novel drug candidates for the treatment of neurological diseases. Three of the most advanced programs include:
MGlu7 NAM for stress related disorders, including PTSD. The company is developing mGlu7 NAM as a novel orally available treatment to reduce fear memory in PTSD, a disorder that can lead to intense fear and anxiety. Subject to regulatory approval, the company's mGlu7 NAM may offer an innovative and differentiated treatment approach from existing therapies. The program has completed clinical candidate selection phase and the company expects to initiate IND enabling studies in the second half of 2023.
MGlu2 NAM for the treatment of mild neurocognitive disorders, or mNCD. The company is developing mGlu2 NAM as a novel orally available treatment for mNCD associated with Alzheimer's disease, Parkinson's disease and depressive disorders. The program has entered clinical candidate selection phase and the company expects to enter IND enabling studies in 2024.
M4 PAM for the treatment of schizophrenia and other psychosis. The company is developing M4 PAM as a novel orally available treatment for schizophrenia and other psychosis. The company is optimizing multiple chemical series of highly selective M4 PAMs compounds and expect to enter into clinical candidate selection phase in late 2023.
Based on the company's expertise in allosteric modulation, the company's goal is to build a leading neuroscience company focused on conditions where current treatment options are limited and where unmet medical needs exist. The company's business strategy includes the possibility of entering into collaborative arrangements with third parties to complete the development and commercialization of the company's proprietary drug candidates, such as the company's partnership with Janssen for ADX71149 and the company's strategic partnership with Indivior for GABAB PAM. The company cannot forecast with any degree of certainty which proprietary products or indications, if any, will be subject to future collaborative arrangements, in whole or in part, and how such arrangements would affect the company's development plan or capital requirements. To date, the company has secured grants and other funding from: The Michael J. Fox Foundation for Parkinson's Research, or MJFF, for the development of dipraglurant for the treatment of PD-LID; the National Institute of Drug Abuse, or NIDA, to generate important data on the role of GABAB in substance use disorder; the Swiss Innovation Agency, or Innosuisse, to advance the company's understanding of the role of the company's drug candidates in neurodegenerative and psychiatric diseases; the Eurostars Joint Programme, or Eurostars to identify novel drug candidates on mGlu7 NAM for PTSD; and the Charcot-Marie-Tooth Association, or CMTA to evaluate the role of GABAB PAM compounds in preclinical models of CMT1A. As the company advances its clinical and preclinical programs, the company will continue to apply for subsidies, grants and government or agency sponsored studies that could offset or reduce the company's development costs.
Research and Development Portfolio
Using the company's allosteric modulator platform and drug discovery and development expertise, the company has established a portfolio of clinical and preclinical programs, internally and with partners.
Externally Developed Out-licensed Drug Candidate
ADX71149, mGlu2 PAM for the treatment of epilepsy. The company's partnered drug candidate, ADX71149 is a novel orally active mGlu2 PAM. The company's partner, Janssen, has completed Phase 1 and two Phase 2a clinical trials in schizophrenia and anxious depression, respectively. Janssen has conducted several preclinical studies in epilepsy and is conducting a placebo-controlled Phase 2a proof of concept clinical trial of ADX71149 in epilepsy patients since June 2021 and an open label study since the third quarter of 2022. Part 1 of the study has been completed and an IRC review to determine the next steps for the program is expected at the beginning of the second quarter of 2023. Under the company's agreement with Janssen, Janssen is responsible for financing the development and commercialization, if any, of ADX71149.
Internally Developed Drug Candidates
Dipraglurant, mGlu5 NAM under evaluation for future development. The company's lead drug candidate, dipraglurant, is a novel orally active mGlu5 NAM under evaluation for future development in a range of potential therapeutic indications, including PD-LID, post-stroke recovery, SUD and pain. As part of this evaluation, the company has initiated discussions with potential partners with the objective of collaborating with a potential strategic partner for the future development of dipraglurant.
The company received orphan drug designation from the United States Food and Drug Administration, or FDA, for dipraglurant in PD-LID and completed a Phase 2 proof of concept study. On June 17, 2022, the company terminated its U.S. registration program, including a pivotal Phase 2B/3 study and an open label study in PD-LID due to slow recruitment of patients.
Material Internal Research Programs
GABAB PAM for the treatment of substance use disorder. The company's partner, Indivior, has licensed worldwide rights to the company's GABAB PAM program and is responsible for all development, manufacture and commercialization of any selected GABAB PAM drug candidates. Under the agreement, the company is responsible for executing a research program funded by Indivior to discover novel drug candidates. Indivior has the right to select GABAB PAM drug candidates from the company's research program. The company is in clinical candidate selection phase and expect IND enabling studies to be initiated in 2024. Indivior's primary focus is substance use disorder. Substance use disorder is an indication with a significant commercial opportunity. Existing therapies often do not provide effective control of symptoms or have side effects that discourage adherence. Subject to regulatory approval, GABAB PAM may offer an innovative and differentiated treatment approach from existing therapies and may provide benefit to patients.
GABAB PAM for the treatment of CMT1A, chronic cough and pain. The company's license agreement with Indivior provides for a funded research program, under which the company has the right to select drug candidates for exclusive development in certain indications outside of SUD. The company plans to develop the company's selected drug candidates in CMT1a, chronic cough and pain. These indications have been validated with baclofen, an orthosteric agonist of GABAB and present a significant unmet medical need and commercial opportunity. The company is in clinical candidate selection phase and expect IND enabling studies to be initiated in 2024.
mGlu7 NAM for the treatment of stress related disorders including PTSD. The company is developing mGlu7 NAM as a novel orally available treatment to reduce fear memory in PTSD, a disorder that can lead to intense fear and anxiety. By selectively targeting mGlu7 with NAMs, the brain circuitries involved in fear and anxiety can be more precisely modulated, potentially resulting in a more focused response and fewer side effects than current therapeutic approaches. Subject to regulatory approval, the company's mGlu7 NAM may offer an innovative and differentiated treatment approach from existing therapies. The company has selected a drug candidate and the company expects to initiate IND enabling studies in the second half of 2023.
mGlu2 NAM for the treatment of mild neurocognitive disorders, or mNCD. The company is developing mGlu2 NAM as a novel orally available treatment for mNCD associated with Alzheimer's disease, Parkinson's disease and depressive disorders. The program has entered clinical candidate selection phase and the company expects to enter IND enabling studies in 2024.
Early-Stage Internal Research Programs
M4 PAM for the treatment of Schizophrenia and Psychosis. The company is developing M4 PAM as a novel orally available treatment for schizophrenia and other psychoses. The company is optimizing multiple chemical series of highly selective M4 PAMs with compounds in late lead optimization.
mGlu4 PAM for the treatment of Parkinson's disease. The company is developing mGlu4 PAM as a novel orally available treatment for Parkinson's disease. The company is optimizing multiple chemical series of highly selective mGlu4 PAMs with compounds in early lead optimization.
mGlu3 PAM for the treatment of neurodegenerative disorders. The company is developing mGlu3 PAM as a novel orally available treatment for neurodegenerative disorders. The company is optimizing multiple chemical series of highly selective mGlu3 PAMs, with compounds in early lead optimization.
Strategy
The key elements of the company's strategy are to pursue additional indications for dipraglurant; continue to advance the company's GABAB PAM research programs; continue to advance the company's mGlu7 NAM program; continue to advance the company's mGlu2 NAM program; continue to advance the company's preclinical programs; continue to pursue collaborative arrangements with other pharmaceutical companies; and leverage the company's expertise and experience in allosteric modulation.
Intellectual Property
Patents and Proprietary Rights
As of December 31, 2022, the company owned 12 U.S. and 179 foreign patents and a number of pending patent applications that cover various aspects of the company's allosteric modulator technologies and discovery platform, including several classes of compounds, which are potentially useful as modulators of mGlu5, mGlu2, mGlu4, mGlu7, and GABAB. More specifically, the company's patents and patent applications cover compounds, pharmaceutical compositions, polymorphs and uses of compounds for medical treatment.
The company typically files priority applications at the United Kingdom Patent Office to establish a priority date for the generic subject matter and examples which are available at the filing date of each invention. Subsequently, the company files international applications under the Patent Cooperation Treaty or PCT, with extra examples to support the scope of the claims (International Phase). After the International Phase, the company files patent applications in selected countries representing potential major markets for the company's drug candidates (National/Regional Phase).
The company has two patent families covering dipraglurant as a composition of matter and its polymorphs which are useful as mGlu5 NAMs: 111 patents have been granted to the company, including 4 in the United States and 97 in other international jurisdictions (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Great Britain, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Monaco, the Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland/Liechtenstein, Turkey, Armenia, Australia, Brazil, Canada, China, Hong Kong, Indonesia, Israel, Japan, South Korea, Mexico, New Zealand, the Philippines, Russia, Singapore, South Africa and Ukraine). The company also has 4 patent applications pending.
The company has one patent family covering compounds which are useful as GABAB PAMs, of which 24 patents have been granted, including 2 in the United States and 22 in other international jurisdictions (Austria, Belgium, Denmark, Finland, France, Germany, Great Britain, Ireland, Italy, the Netherlands, Norway, Spain, Sweden, Switzerland/Liechtenstein, Australia, Canada, China, India, Israel, Japan, and New Zealand).
Jointly with Janssen, the company has 69 patents in three patent families covering compounds which are useful as mGlu2 PAMs, including ADX71149, which is explicitly exemplified and claimed as a compound and as a pharmaceutical composition, the company has 4 patents in the United States and 65 in other international jurisdictions (Albania, Austria, Belgium, Bosnia & Herzegovina, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, North Macedonia, Malta, Monaco, the Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland/Liechtenstein, Turkey, Ukraine, United Kingdom, Algeria, Argentina, Australia, Brazil, Canada, China, Chile, Gulf Cooperation Council, Hong Kong, Israel, India, Indonesia, Japan, Republic of Korea, Malaysia, Mexico, New Zealand, the Philippines, Russia, Singapore, South Africa, Thailand, Vietnam, and Taiwan).
Furthermore, the company has 9 patents in two patent families covering compounds that have potential utility as mGlu4 PAMs, which include 2 patents granted in the United States and 7 in Europe (Belgium, France, Germany, Great Britain, Italy, Spain and Switzerland/Liechtenstein). One family is owned by the company and a second family is jointly owned by the company and Merck & Co Inc. pursuant to the company's collaboration agreement for the development of mGlu4 PAM, which the company entered into in 2007.
In November 2022, 2 PCT applications for mGlu7 NAM project were published.
The company's portfolio of granted patents have expiry dates ranging from 2025 - 2034 without extension. Patent term extension of up to 5 years is available in some jurisdictions. For example, in the U.S. following the enactment of Title II of the Drug Price Competition and Patent Term Restoration Act (Public Law 98-417) it is generally possible to extend patent life by a maximum of 5 years.
Trademarks
The company owns trademarks for Addex Pharmaceuticals in Switzerland.
History
Addex Therapeutics Ltd was founded in 2002.

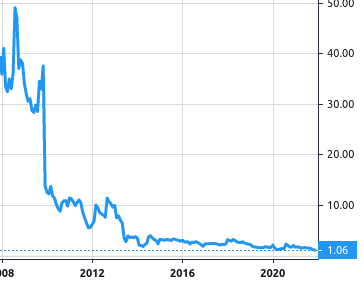
 Stock Value
Stock Value

