About International Stem Cell
International Stem Cell Corporation (ISCO) operates as a clinical stage biotechnology company.
The company focuses on therapeutic and biomedical product development with multiple long-term therapeutic opportunities and two revenue-generating businesses offering potential for increased future revenue.
The company has no revenue generated from the company's principal operations in therapeutic and clinical product development through research and development efforts. The company has generated revenue from the company's two commercial businesses, anti-aging and research products.
The company's products are based on multi-decade experience with human cell culture and a proprietary type of pluripotent stem cells, 'human parthenogenetic stem cells' ('hpSCs'). The company's hpSCs are comparable to human embryonic stem cells ('hESCs') in that they have the potential to be differentiated into many different cells in the human body. However, the derivation of hpSCs does not require the use of fertilized eggs or the destruction of human embryos and also offers the potential for the creation of immune-matched cells and tissues that are less likely to be rejected following transplantation. ISCO scientists have created the first parthenogenetic, homozygous stem cell line that can be a source of therapeutic cells for hundreds of millions of individuals with minimal immune rejection after transplantation. The company has facilities and manufacturing processes that comply with the requirements of current Good Manufacturing Practice ('GMP') standards as defined by the U.S. Code of Federal Regulations and promulgated by the Food and Drug Administration ('FDA').
The company is developing different cell types from the company's stem cells that may result in therapeutic products. The company focuses on applications where cell and tissue therapy are already proven but where there is an insufficient supply of functional cells or tissue. The most promising potential clinical application of the company's technology is for neural stem cells (ISC-hpNSC) for the treatment of Parkinson's disease and potentially other central nervous system disorders, such as traumatic brain injury, stroke and Alzheimer's disease.
The company's most advanced project is the neural stem cell program for the treatment of Parkinson's disease. In 2013, the company published in Nature Scientific Reports the basis for the company's patent on a new method of manufacturing neural stem cells which is used to produce the clinical-grade cells necessary for future clinical studies and commercialization. In 2014, the company completed the majority of the preclinical research establishing the safety profile of neural stem cells ('NSC') in various animal species, including non-human primates. In June 2016, the company published the results of a 12-month pre-clinical non-human primate study that demonstrated the safety, efficacy and mechanism of action of the ISC-hpNSC. In 2017, the company began its Phase I trial of ISC-hpNSC, human parthenogenetic stem cell-derived neural stem cells for the treatment of Parkinson's disease. This trial involves three groups, each with four patients, with each group receiving an increasing amount of ISC-hpNSC via intracerebral transplantation. Patients are evaluated for 12 months (active phase of the study) with an additional 5-year observational follow-up period to assess safety. The company reported 12-month results from the first cohort and 6-month interim results of the second cohort at the Society for Neuroscience annual meeting (Neuroscience 2018) in November 2018. In April 2019, the company announced the completion of subject enrollment, with the 12th subject receiving a transplantation of the highest dose of cells. There have been no safety signals or serious adverse effects seen to date as related to the transplanted ISC-hpNSC cells. The company announced successful completion of the dose escalating phase 1 clinical trial in June 2021. In terms of preliminary efficacy, where scores are compared against baseline before transplantation, the company observed a potential dose-dependent response, with an apparent peak effectiveness at the company's middle dose. The % OFF-Time, which is the time during the day when levodopa medication is not performing optimally and PD symptoms return, decreased an average 47% from the baseline at 12 months post transplantation in cohort 2. This trend continued through 24 months where the % OFF-Time in the second cohort dropped by 55% from the initial reading. The same was true for % ON-Time without dyskinesia, which is the time during the day when levodopa medication is performing optimally without dyskinesia. The % ON-Time increased an average of 42% above the initial evaluation at 12 months post-transplantation in the second cohort.

The company also has licensed worldwide rights to use a technology known as Somatic Cell Nuclear Transfer ('SCNT') to create human stem cells. Countries, such as the United Kingdom have made similar recommendations.
Platform Technology
The company has developed a proprietary process based on parthenogenesis for the creation of a new type of stem cell that has shown to exhibit the pluripotency and proliferative benefits of embryonic stem cells yet avoid the use or destruction of fertilized human eggs or embryos.
The company also holds licenses to three other technologies to create human pluripotent stem cells: SCNT technology (as mentioned previously); a technology that may be useful to create induced pluripotent stem cells ('iPS'); and 'single blastomere technology', which uses a single cell obtained from a fertilized blastocyst to create an embryonic stem cell line. Each of these technologies has unique cell therapy applications and provides the company with a broad base of technologies from which the company can operate in the future.
Products
Therapeutic Product Candidates
The company is developing different cell types from the company's stem cells that may result in therapeutic products. The company focuses on applications where cell and tissue therapy is already proven but where there is an insufficient supply of functional cells or tissue.
The most promising potential clinical applications of the company's technology are Parkinson's disease ('PD'), traumatic brain injury ('TBI'), and stroke. Using the company's proprietary technologies and know-how, the company is creating neural stem cells from hpSCs as a potential treatment of PD, TBI, and stroke.
PD: The company's most advanced project is the neural stem cell program for the treatment of Parkinson's disease. In 2013, the company published in Nature Scientific Reports the basis for the company's patent on a new method of manufacturing neural stem cells, which is used to produce the clinical-grade cells necessary for future clinical studies and commercialization. In 2014, the company completed the majority of the preclinical research, establishing the safety profile of NSC in various animal species, including non-human primates. In June 2016, the company published the results of a 12-month pre-clinical non-human primate study, which demonstrated the safety, efficacy and mechanism of action of the ISC- hpNSC. In 2017, the company dosed four patients in the company's Phase I trial of ISC-hpNSC, human parthenogenetic stem cell-derived neural stem cells for the treatment of Parkinson's disease. The company reported 12-month results from the first cohort and 6-month interim results of the second cohort at the Society for Neuroscience annual meeting (Neuroscience 2018) in November 2018. In April 2019, the company announced the completion of subject enrollment, with the 12th subject receiving a transplantation of the highest dose of cells. There have been no safety signals or serious adverse effects seen to date as related to the transplanted ISC-hpNSC cells.
The company announced a successful completion of the dose escalating phase 1 clinical trial in June 2021. In terms of preliminary efficacy, where scores are compared against baseline before transplantation, the company observed a potential dose-dependent response with an apparent peak effectiveness at the company's middle dose.
Stroke: In August 2014, the company announced the launch of a stroke program, evaluating the use of ISC-hpNSC transplantation for the treatment of ischemic stroke using a rodent model of the disease. The company has a considerable amount of safety data on ISC-hpNSC from the Parkinson's disease program and, as there is evidence that transplantation of ISC-hpNSC may improve patient outcomes as an adjunctive therapeutic strategy in stroke, having a second program that can use this safety dataset is therefore a logical extension. In 2015, the company together with Tulane University demonstrated that NSC can significantly reduce neurological dysfunction after a stroke in animal models.
TBI: In October 2016, the company announced the results of the pre-clinical rodent study, evaluating the use of ISC-hpNSC transplantation for the treatment of TBI. The study was conducted at the University of South Florida Morsani College of Medicine. The company demonstrated that animals receiving injections of ISC-hpNSC displayed the highest levels of improvements in cognitive performance and motor coordination compared to vehicle control treated animals. In February 2019, the company published the results of the pre-clinical study in Theranostics, a prestigious peer-reviewed medical journal. The publication titled, 'Human parthenogenetic neural stem cell grafts promote multiple regenerative processes in a traumatic brain injury model,' demonstrated that the clinical-grade neural stem cells used in the company's Parkinson's disease clinical trial, ISC-hpNSC, significantly improved TBI-associated motor, neurological, and cognitive deficits without any safety issues.
Each of these product candidates will require extensive preclinical and clinical development and may require specific unforeseen licensing rights obtained at substantial cost before any regulatory approval may be achieved and the products sold for therapeutic use.
Anti-Aging Skin Care Products
ISCO's wholly owned subsidiary Lifeline Skin Care, Inc. ('LSC') develops, manufactures and sells anti-aging skin care products based on two core technologies: encapsulated extract derived from hpSC and specially selected targeted small molecules. As of December 31, 2022, LSC's products included ProPlus Advanced Defense Complex; ProPlus Advanced Recovery Complex; ProPlus Eye Firming Complex; ProPlus Neck Firming Complex; ProPlus Advanced Aqueous Treatment; ProPlus Collagen Booster (Advanced Molecular Serum); ProPlus Elastin Booster; and ProPlus Brightening Toner.
LSC's products are regulated as cosmetics. LSC's products are sold domestically through a branded website, Amazon, and ecommerce partners.
Research Products
ISCO's LCT subsidiary develops, manufactures and commercializes over 200 human cell culture products. These products include frozen human 'primary' cells and stem cells and the reagents (called 'media') needed to grow, maintain and differentiate the cells. LCT's scientists have used a technique called basal medium optimization to systematically produce optimized products designed to culture specific human cell types and to elicit specific cellular behaviors. These techniques also produce products that do not contain non-human animal proteins, a feature desirable to research and therapeutic markets. These human cell-based products are used domestically and internationally by research scientists in pharmaceutical, academic and government research organizations to study human disease and basic cell biology. LCT's products eliminate the need for scientists to create their own cells, media and reagents or attempt to adapt 'off the shelf' products to match specific experimental needs and they are superior to using animals or non-human animal cells as research tools because they are more relevant to the study of human disease. Strict quality assurance provides a high level of consistency and standardization of these products. LCT offers products that contain no animal products ('called 'Xeno-free' products), allowing researchers to have better control of their experiments and to conduct research using products that ultimately can be more appropriate for therapeutic applications.
Often LCT's research customers use the company's cell-based research products in their clinical research, eventually adapting them for therapeutic applications. If one of the company's research products is adopted by a successful producer of therapeutic cells, ISCO may become a supplier to the much larger therapeutic market through LCT's products. This is based on the fact that once regulatory product submissions are made to the FDA and similar authorities, the media and reagents used during development cannot be changed easily after approval. These uses of LCT's products bring opportunities to ISCO for future therapeutic products.
LCT products and applications include:
Human skin cells and associated reagents for the study of skin disease, toxicology or wound healing.
Human cells from the heart and blood vessels and associated reagents (VascuLife ), used by researchers to study cardiovascular disease and cancer.
Human bronchial and tracheal cells for the study of toxicity, cystic fibrosis, asthma and pathogenesis.
Human mammary epithelial cells for the study of breast cancer, three dimensional culture and carcinogen screening.
Adult stem cells (called mesenchymal stem cells) and the reagents necessary to differentiate them into various tissues, including bone, cartilage and fat. These products are valuable for researchers in the emerging field of regenerative medicine.
Human prostate cells and specialized medium (ProstaLife) to study prostate disease including cancer.
Human renal and bladder cells and associated media (RenaLife) to study renal and bladder diseases.
Human corneal cells and associated media (OcuLife) for the study of corneal disease and as a model of toxicology for consumer product testing.
Human female reproductive system cells (ReproLife) for the study of cellular physiology of the reproductive tract, cellular response to infectious agents and other areas of female reproductive system research.
Human Skeletal Muscle Cells (StemLife Sk) for the study of muscle cell biology, diabetes, insulin receptor studies, muscle metabolism, muscle tissue repair and myotube development.
An assortment of many other cell culture reagents and supplements for the growth, staining and freezing of human cells.
Each LCT cell product is quality tested for the expression of specific markers (to assure the cells are the correct type), proliferation rate, viability, morphology and absence of pathogens. Each cell system also contains associated donor information and all informed consent requirements are strictly followed.
LCT's research products are marketed and sold by its internal sales force, LCT brand distributors in Europe and Asia and original equipment manufacturing (OEM) partners, which are then re-branded and sold with OEM partners' labels.
Markets
Therapeutic Markets
ISCO is pursuing a number of scientific development programs designed to lead to the creation of new therapeutic products. The company anticipates that, with their superior immune-matching characteristics, the company's cells will be able to reduce or eliminate the need for immune-suppression drugs and the adverse reactions they trigger in patients.
Parkinson's Disease. Using the company's proprietary technologies and know-how, the company is creating neural stem cells from hpSCs as a potential treatment of PD and potentially other central nervous system disorders, including traumatic brain injury, in order to address this significant market opportunity.
Intellectual Property
Patents
In 2022, ISCO was issued one patent for technology generated by the company's R&D team. The patent, issued in the USA, covers the use of Parthenogenic Activation of Human Oocytes. As of December 31, 2022, the company held a total of 39 patents. These patents expire from June 2025 through June 2037.
In addition, the company has obtained exclusive worldwide licenses to patents and patent applications from Astellas Pharma.
The majority of the patents and applications have been filed in the U.S. and in foreign countries through the Patent Cooperation Treaty or by direct country filings in those jurisdictions deemed significant to the company's operations.
The company has protected its research products and branding through both patents and trademarks. Lifeline Skin Care has filed patent applications covering its proprietary core technologies and methods of using stem cells and targeted small molecules to create skin care products. LSC unique product formulas are protected as trade secrets. ISCO, LCT, and LSC have registered trademarks on their company names, logos and various product names to protect their branding investment. Lifeline Cell Technology's reagent formulations are protected as trade secrets.
License Agreements
In May 2005, the company entered into three exclusive license agreements ('ACT IP,' 'Infigen IP,' and 'UMass IP' or collectively 'ACTC agreements') with Astellas Pharma Inc. ('Astellas') for the production of therapeutic products in the fields of diabetes, liver disease, retinal disease and the creation of research products in all fields. In February 2013, each of these license agreements was amended and restated, pursuant to which the company continues to have rights to Astellas Pharma's human cell patent portfolio and non-exclusive rights to future developments in the area of diabetes and liver disease, as well as certain rights to patents covering Single Blastomere technology.
Research Agreements
ISCO actively pursues sponsored research agreements with local and international research organizations and has established research collaborations with collaborators from Yale University, University of South Florida, Tulane University, University of California, San Diego, The Scripps Research Institute (La Jolla), and the Sanford Burnham Preby Medical Discovery Institute.
Competition
Some of the company's primary competitors in the development of stem cell therapies are BioTime, SanBio, BlueRock Therapeutics, and ReNeuron. The company's primary competitors in the skin care market are Obagi, ZO Skin Health, Skinceuticals, SkinMedica (now owned by Allergan), and Murad. In the field of research products, the company's primary competitors for human cells, media and reagents are Lonza, EMD Millipore, Life Technologies (now owned by Thermo Fisher Scientific), StemCell Technologies, Zen-bio, PromoCell, and Specialty Media.
Sales and Marketing
As of December 31, 2022, sales of the company's research products had been derived primarily through its in-house sales force and via OEM partners and LCT brand distributors in Europe and Asia. Approximately 45% of the company's total product sales in 2022 were from one customer.
LSC phased out its retail product line in 2019, with the exception of select cleanser products that were offered to both professional and retail customers. LSC is now offering its ProPLUS product line through its branded website - www.lifelineskincare.com, as well as through a network of select online retailers and a limited number of professional accounts, such as dermatologists, and plastic surgeons. Domestically, the company plans to increase distribution of the company's products through increasing brand awareness, strategic partnerships, and sales promotions.
Government Regulation
The company has made extensive progress in obtaining the necessary regulatory approvals of research protocols, informed consent documents and donor protection procedures to obtain oocytes in the United States for the production of the company's parthenogenetic stem cell bank. These approvals include: federally mandated Institutional Review Board (IRB) and State of California required Stem Cell Research Oversight (SCRO) committee.
Research and Development Expenses
The company's research and development expenses were $492 thousand for the year ended December 31, 2022.
History
International Stem Cell Corporation was founded in 2001.

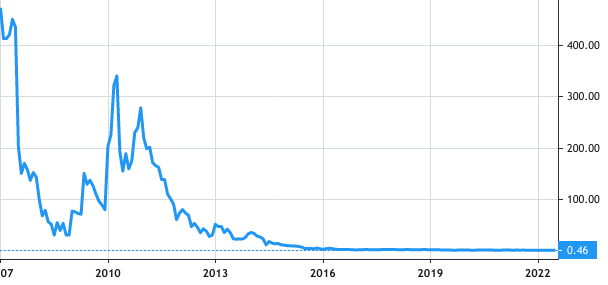
 Stock Value
Stock Value

