About Tenaya Therapeutics
Tenaya Therapeutics, Inc. operates as a clinical-stage biotechnology company. The company develops and delivers potentially curative therapies that address the underlying drivers of heart disease.
The company’s understanding of the links between heart disease and genetic factors is increasing exponentially, creating new opportunities for the advancement of novel disease-modifying therapeutics that target the underlying cause of disease. The company is advancing a deep and diverse pipeline of therapeutic programs intended for both rare and highly prevalent forms of heart disease. Each of the company’s lead product candidates, TN-201, a gene therapy for myosin binding protein C3 (MYBPC3)-associated hypertrophic cardiomyopathy (HCM), TN-301, a small molecule for heart failure with preserved ejection fraction (HFpEF), and TN-401, a gene therapy for plakophilin 2 (PKP2)-associated arrhythmogenic right ventricular cardiomyopathy (ARVC), emerged from its proprietary integrated drug discovery platforms and has progressed to the clinic or late-stage preclinical development with the support of its core internal capabilities.
In addition to its lead product candidates, the company has multiple early-stage programs progressing through pre-clinical development. These programs include an adeno-associated virus (AAV)-based gene therapy designed to express the Dwarf Open Reading Frame (DWORF) gene in the heart with potentially broad utility in dilated cardiomyopathy (DCM), as well as its reprogramming program for cardiac regeneration which aims to replace heart cells lost in patients experiencing heart failure due to prior myocardial infarction (MI). While these named programs have reached candidate selection stage, the company also has numerous earlier-stage programs emerging from its proprietary product platforms to address other forms of heart failure.
The company’s distinct, but interrelated Gene Therapy, Cellular Regeneration and Precision Medicine platforms and suite of integrated capabilities support its efforts to discover disease-modifying treatments focused on heart disease in a modality-agnostic manner. The company also continues to invest in complementary new technologies and the optimization of its existing proprietary capabilities, including the use of human-induced Pluripotent Stem Cell (iPSC) disease models, machine learning and phenotypic screening, capsid engineering and novel promoter constructs to enable the discovery, design, delivery and development of therapeutics that are best suited to a given cardiovascular condition. In 2022, the company also launched operations of its Genetic Medicines Manufacturing Center (GMMC) based in Union City, CA. The facility utilizes a modular, scalable design to produce AAV-based gene therapies under current Good Manufacturing Practice (cGMP) standards.
TN-201
TN-201 is the company’s potential first-in-class gene therapy for adults and children with HCM due to MYBPC3 gene mutations, the most common cause of familial HCM. TN-201 has received orphan drug designation from the FDA and orphan medicinal product designation from the European Commission (EC). In January 2023, the company received notification from the FDA that clinical testing of TN-201 may proceed, and in the third quarter of 2023, it expects to begin dosing patients in a Phase 1b multi-center, open-label clinical trial, designed to assess the safety, tolerability and efficacy of a one-time intravenous infusion of TN-201. Data from the trial is anticipated in 2024.

TN-201 is an AAV-based gene therapy designed to deliver a fully functional MYBPC3 gene to restore normal levels of the cardiac MyBP-C protein and in order to halt disease progression and reverse the course of genetic HCM after a single treatment. The company is developing TN-201, a potential first-in-class AAV-based gene therapy designed to deliver a fully functional MYBPC3 gene and to restore normal levels of MyBP-C protein, driven by its proprietary cardiac specific promoter. TN-201 has the potential to address the underlying biological basis of disease in adult and pediatric HCM patients with homozygous or heterozygous MYBPC3 gene mutations.
In January 2023, the company received clearance of its IND from the FDA to conduct a Phase 1b clinical trial of TN-201 in symptomatic adults with the nonobstructive form of MYBPC3-associated HCM. The company expects to commence patient dosing in the Phase 1b in the third quarter of 2023 and has completed all necessary manufacturing of TN-201 to supply the clinical trial.
In preclinical studies, the company systemically administered a mouse surrogate of TN-201 (AAV:mMybpc3 or mTN-201) in two-week-old Mybpc3 KO mice. During optimization of its MYBPC3 gene therapies, the company discovered a cardiomyocyte-specific promoter, TNP-CM1, with improved performance attributes as compared to the standard cardiac troponin T (cTnT) promoter. In vitro and in vivo analyses confirmed that TNP-CM1 significantly increased expression of the MYBPC3 gene compared to what can be achieved with the standard cTnT promoter.
TN-301
TN-301 is the company’s highly specific small molecule inhibitor of histone deacetylase 6 (HDAC6). TN-301 is initially being developed for the potential treatment of HFpEF. HFpEF is characterized by a stiffening of the heart muscle resulting in an inability for the left ventricle (LV) to relax properly during normal heart rhythm, referred to as diastolic dysfunction. The company is conducting a Phase 1 clinical trial in healthy adult participants to evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of escalating oral doses of TN-301. The Phase 1 clinical trial is being conducted in two stages: a single-ascending dose (SAD) stage and a multiple-ascending dose (MAD) stage. Data from both the SAD and MAD stages of the trial are anticipated in the second half of 2023.
The company is developing TN-301, a highly specific small molecule inhibitor of HDAC6 for the potential treatment of HFpEF. HDAC6 is localized to the cell cytoplasm where it interacts with multiple proteins to coordinate cellular processes. In animal models intended to mimic human HFpEF, its highly selective HDAC6 inhibitors reversed preexisting cardiac hypertrophy and diastolic dysfunction, and improved lung congestion and exercise capacity, all of which are hallmarks of HFpEF.
TN-301 and the company’s related HDAC6 inhibitors were discovered using its distinct Precision Medicine targeted drug discovery platform technologies, involving phenotypic screening and deep learning to human iPSCs. In vitro, the company’s HDAC6 inhibitors demonstrated up to 2500-fold preferential selectivity for HDAC6, reduced sarcomeric damage and enhanced cardiac energetics. In in vivo studies in multiple mouse models of HFpEF, TN-301 and TYA-018, a structurally and functionally equivalent compound used for preclinical testing, demonstrated reductions in inflammation and metabolic dysfunction, as well as decreased fibrosis, hypertrophy and diastolic dysfunction.
The company is conducting a randomized (3:1), double-blind, placebo-controlled Phase 1 clinical trial designed to assess the safety and tolerability of escalating oral doses of TN-301 in healthy adult participants. Secondary objectives of the clinical trial will be to assess PK and PD measures. The trial is being conducted in two stages. In the first stage, participants receive single ascending doses of either TN-301 or placebo and based on data from the SAD stage of the trial, including PD evidence of target engagement, participants in the second stage receive multiple ascending doses of TN-301 at dose levels of interest to help guide dosing in future trials. Dosing in the SAD stage of the trial began in September 2022. In addition to improvements in glucose metabolism associated with TN-301 treatment in HFpEF mouse models, treatment with TN-301 has also led to improvements in glucose tolerance and insulin sensitivity in a DIO mouse model.
Through its target identification Precision Medicine platform, HDAC6 was initially identified as a target for a genetically defined subset of DCM, BAG3 mutant DCM. The company screened a large chemical library to identify compounds able to reverse sarcomere defects in BAG3-deficient human iPSC-CMs. Sarcomere defects were rapidly and systemically assessed through the company’s proprietary machine learning algorithms. The company has validated these in vitro findings by testing its HDAC6i compounds in BAG3 mutant mice models.
The company is developing TN-401, a potential first-in-class AAV-based gene therapy designed to address ARVC caused by PKP2 gene mutations. The company has demonstrated prevention of disease progression, reversal of RV remodeling and survival benefit in a murine model after a single dose. The company expects to submit an IND to the FDA in the second half of 2023, and those efforts are being aided by its learnings from the TN-201 IND filing experience.
TN-401
The company is developing a potential first-in-class AAV-based gene therapy, TN-401, designed to deliver a functional PKP2 gene in adults with ARVC due to a PKP2 genetic mutation. PKP2-associated ARVC is estimated to affect more than 70,000 patients in the U.S. PKP2 mutations can cause enlargement of the right ventricle (RV) in affected individuals, replacement of heart muscle with fibrotic tissue and fatty deposits, and severely abnormal heart rhythms (arrhythmia) that can make it harder for the heart to function properly and result in sudden cardiac death in some adults and children. The company’s product candidate, TN-401, has demonstrated prevention of disease progression and survival benefit after a single dose in a mouse model of ARVC, as well as tolerability in a pilot non-Good Laboratory Practices (GLP) toxicology and biodistribution study. TN-401 has received orphan drug designation from the FDA. The company has initiated investigational new drug application (IND)-enabling studies for TN-401 and expects to submit an IND to the FDA in the second half of 2023 to enable clinical development of TN-401.
Early-Stage Research Efforts
In addition to its lead product candidates, the company has multiple early-stage programs progressing through preclinical development, including an AAV-based gene therapy designed to express the Dwarf Open Reading Frame (DWORF) gene in the heart with potentially broad utility in DCM and a cellular reprogramming program for cardiac regeneration which aims to replace heart cells lost due to prior MI. The company’s researchers are conducting preclinical testing of these and several other genetically targeted leads emerging from its proprietary product platforms to address other forms of heart failure. In addition to its novel drug discovery efforts, the company continues to invest in the further development of its gene therapy-enabling technologies and capabilities.
Product Platforms
The company has established three distinct but interrelated product platforms -- Gene Therapy, Cellular Regeneration and Precision Medicine -- to discover novel therapies for various forms of heart disease. These platforms bring together differentiated science, capabilities, and intellectual property to enable multi-modality drug discovery.
The company’s Gene Therapy platform uses AAVs to deliver healthy genes to specific cells in the heart to correct or compensate for functional defects. While its lead gene therapy programs utilize AAV9 to deliver healthy genes to the heart, the company has the ability to use both known AAV capsids, as well as novel capsids identified through its internal capsid engineering capabilities. In the future, the company may also explore other delivery options, including non-viral delivery.
The company’s Cellular Regeneration platform uses viral vectors to deliver specific combinations of genes to existing cells in the heart to regenerate cardiomyocytes through two distinct in vivo approaches: One approach uses AAV vectors to deliver proprietary combinations of genes that induce the resident cardiac fibroblasts to convert to cardiomyocytes. Another approach uses non-integrating lentiviruses to deliver proprietary combinations of genes that induce the resident cardiomyocytes to undergo transient cell division. The product candidates arising from this platform are intended to overcome the shortcomings of traditional therapies that address symptoms but are not able to address the irreversible loss of cardiomyocytes.
The company’s Precision Medicine platform for target identification uses human genetic information combined with phenotypic high throughput screening in its proprietary human iPSC-CM models of human disease and machine learning algorithms for the identification and validation of novel targets for heart diseases.
Core Capabilities
Foundational to its research and drug discovery efforts are the company’s proprietary integrated core capabilities that collectively support discovery of novel targets, in vitro optimization and validation of leads, rapid product development, precise product delivery, and efficient production, which ultimately improves the probability of technical and regulatory success of its product candidates.
The company’s five core capabilities include:
Disease Models: The company has internalized the ability to create and integrate proprietary in vitro and in vivo models within its research organization, as existing models of human heart disease may not be adequate to assess the efficacy or safety of novel therapies. The company’s disease modelling capabilities serve to facilitate the discovery of new leads and to characterize the activity of existing leads as it moves through preclinical development. For its in vitro human iPSC-CM disease models, the company uses multiple methods to induce phenotypes within cell lines that simulate human diseases and then use these models for high throughput target identification and drug discovery. For its in vivo disease models, the company has a dedicated onsite in vivo pharmacology group and vivarium, where it has established approximately 17 rodent heart disease models, both genetic and non-genetic, and can dose animals, perform heart surgeries, and use non-invasive imaging to assess the impact of its therapies under development.
Capsid Engineering: The company has established in-house AAV capsid engineering capabilities and have successfully screened over one billion variants from more than 30 diverse, proprietary AAV libraries in multiple in vitro, in vivo, and in silico models to discover novel AAV capsids that can target the different types of cells in the heart. The company has generated preclinical data to support the superiority of these capsids over parental variants in multiple species against multiple attributes to assess their potential to translate across species and into humans. The company’s next-generation capsids are designed to have desirable properties, including the ability to more selectively target the heart versus other organs, as well as lower susceptibility to neutralizing antibodies.
Promoters and Regulatory Elements: The company has created novel promoters and regulatory elements that support its gene therapy and cellular regeneration programs by controlling the expression of genes within the cells. The company uses these innovations to help ensure more precise and more robust expression of therapeutic payloads in the different cell types of the heart as compared to what can be achieved with available methods.
Drug Delivery: The company is actively exploring different routes of administration (ROAs), as well as different infusion- and injection-based methods for delivering its AAV-based therapies. The company has designed a new catheter to support more targeted delivery and more efficient uptake of therapeutic payloads in the heart.
The company is developing a potential first-in-class AAV-based gene therapy to deliver a fully functional copy of the human PKP2 gene to the hearts of ARVC patients carrying PKP2 mutations. The company developed a Pkp2 conditional knockout (Pkp2-cKO) mouse model that simulates key aspects of ARVC, including dilation of the RV, decline in LV heart function, severe arrhythmia, abnormal ECG trace, and early mortality.
The company is also encouraged by the preclinical safety profile and dose-responses observed in its studies of Pkp2-cKO mice following treatment with various doses of TN-401. In these studies, the company observed dose-dependent efficacy against disease attributes, including prevention of LV functional decline, with relatively low doses achieving near maximal efficacy. Single doses of TN-401 administered in Pkp2-cKO mice at the 1×1013 vg/kg, 3×1013 vg/kg and 1×1014 vg/kg dose levels were shown to achieve robust protein expression of PKP2 transgene and desmoplakin (DSP). DSP is one of the five protein components of desmosome in addition to PKP2 and its expression in response to TN-401 is an indicator of improved desmosome integrity following PKP2 protein replacement.
The company has initiated IND-enabling activities and plans to submit an IND to the FDA for TN-401 in the second half of 2023. The company intends to use its experience from the TN-201 IND filing and to seek feedback from multiple regulatory agencies, including the FDA, as necessary. If its IND is approved, the company plans to initiate global FIH clinical trials in patients with mutations of the PKP2 gene. Additionally, in support of its development efforts for TN-401, the company has initiated a global non-interventional study to collect treatment history and seroprevalence to AAV9 antibodies data among ARVC patients who carry pathogenic or likely pathogenic PKP2 gene mutations.
The company is developing an AAV-based gene therapy designed to deliver the DWORF gene for patients with DCM. The company and its academic collaborators have accumulated significant preclinical proof-of-concept evidence for the therapeutic benefit and tolerability of over-expression of the DWORF gene in multiple murine models, including models of DCM.
The company has licensed intellectual property from University of Texas Southwestern Medical Center (UTSW) to develop and commercialize products relating to therapeutics overexpressing DWORF and are developing an AAV-based gene therapy to deliver the DWORF gene to cardiomyocytes for the treatment of DCM. DWORF is a discovered small peptide that localizes primarily to the sarcoplasmic reticulum of the cardiac muscle cell.
The company’s DWORF program is at the candidate selection stage with multiple constructs under consideration. DWORF gene expression is limited to the cardiomyocyte through use of a novel cardiomyocyte-specific promoter. The company’s intended product candidate will use an AAV capsid with high tropism for the heart, either AAV9 or a novel proprietary capsid developed through its capsid engineering capabilities, to deliver the DWORF gene. The company is exploring different ROAs including systemic (IV) or delivery directly to the heart through an infusion catheter.
The company has tested different AAV:DWORF constructs in both healthy and disease mouse models and has not observed any safety signals at clinically relevant levels of DWORF overexpression.
The company is developing an AAV-based approach to cellular regeneration that involves converting (or reprogramming) existing cardiac fibroblasts within the heart to turn into new cardiomyocytes and to replace cells permanently lost due to myocardial infarction (MI). The company has discovered a proprietary combination of three genes that can drive robust in vivo reprogramming of cardiac fibroblasts to cardiomyocytes when delivered together in a single AAV capsid.
The company has conducted in vitro and in vivo experiments to optimize its direct reprogramming approach. The company’s most advanced results have been achieved primarily with two different constructs, TN1-002 and TN1-006.
The company’s preclinical findings to date provide direction to its ongoing candidate selection efforts. The company continues to seek ways to ensure more consistent delivery and expression of its reprogramming factors to cardiac fibroblasts, including with the use of novel capsids and novel delivery methods.
The company has received feedback from the FDA through an INTERACT (INitial Targeted Engagement for Regulatory Advice on CBER producTs) review to inform the design of its future preclinical studies. After selection of its product candidate, the company plans to initiate IND-enabling studies.
The company’s development plan is anticipated to include patients with advanced heart failure due to prior MI who meet qualifications for a heart transplant or LVAD, as well as a broader patient population with severe ischemic cardiomyopathy.
To unlock the full potential of novel therapies across many forms of heart disease, the company is advancing science from three product platforms in parallel. The company is advancing programs from these distinct but interrelated product platforms that combine different science, capabilities, and intellectual property.
The company is advancing a cardiac regeneration approach based on research conducted by its founders at Gladstone Institutes and UTSW, who pioneered the idea of restoring heart function after a heart attack by in vivo regeneration of lost cardiomyocytes.
The company has discovered a proprietary combination of three genes that can be co-packaged and co-expressed from a single proprietary AAV vector engineered for higher transduction of cardiac fibroblasts when compared to existing parental capsids. The company has demonstrated higher transdifferentiation rates in vitro using human cardiac fibroblasts that are higher than rates reported in published studies using combinations of other factors intended to drive reprogramming. The company has demonstrated robust and durable proof-of-concept of this approach in multiple rodent models of acute MI and heart failure post-MI.
The company is conducting target identification screens for both gene therapy and small molecule targets in multiple human iPSC-CM disease models of DCM. The company is also expanding its efforts to different genetic backgrounds including the leading genetic causes of cardiomyopathy.
The company utilizes five core internal capabilities to support its three product platforms. The company’s key capabilities include the creation and development of disease models to more accurately simulate human heart disease phenotypes, proprietary heart-tropic AAV capsids designed to enable precise tissue targeting and increase safety, proprietary promoters and regulatory elements to control gene expression, fit-for-purpose drug delivery methods for more optimal uptake and distribution of its product candidates and scalable AAV manufacturing to better control quality, costs, timelines and supply.
The company has internalized the ability to create and integrate in vitro and in vivo models within its research organization, which allows it to simulate human heart disease phenotypes.
In Vitro Cell-Based Disease Models: For its in vitro disease models, the company has leveraged the seminal discovery of methods used to generate iPSCs to establish disease models based on human iPSC-CMs. The company has implemented three primary approaches to model human heart disease in this way short interfering ribonucleic acid (siRNA) constructs to silence specific genes of interest in iPSC-CMs; CRISPR-based gene editing approaches to create isogenic iPSC-cell lines where specific genes have been altered; and iPSCs derived from patients with severe heart disease, for example, severe DCM resulting in early heart failure and transplant, sourced from commercial and academic collaborators.
The company initially used cells from these disease models plated in two-dimensional formats. The company has since advanced its efforts to include three-dimensional engineered heart tissue disease models where the cells have a more mature phenotype and with contractility that can be measured more reliably.
iPSC production: To conduct robust target identification and drug discovery screens using the company’s cell-based disease models, it need to produce large volumes of these human iPSC-CMs. The company has developed the necessary know-how to do so reliably and reproducibly at increasing scale.
Imaging Techniques: The company uses a combination of immunostaining, high-resolution imaging, and imaging algorithms to visualize and quantify phenotypic differences between its in-house iPSC-CM disease models. The company can measure several details of the sarcomeres of these cell lines, including sarcomere density, disarray and Z-disc area.
Machine Learning Algorithms: The company has used machine learning algorithms to support high-throughput phenotypic screening of its iPSC-CM disease models. The algorithms can rapidly and reproducibly measure subtle differences in the overall appearance between wild-type iPSC-CM cells and the different disease models, as well as differences on the disease models in response to compounds in the company’s screening libraries.
In Vivo Models: For its in vivo disease models, the company has a dedicated onsite in vivo pharmacology group and vivarium. The company has established approximately 17 rodent heart disease models, both genetic and non-genetic, and continue to develop new models in-house as needed. The company can dose both gene therapies, as well as small molecules. The company also works with established CROs for research efforts involving large animal models (e.g., NHPs and pigs), including for efficacy studies and evaluation of drug delivery methods. Through these efforts the company has developed important insights into the advantages and limitations of specific models and have learned how to optimize the design of its experiments. This insight influences the company’s preclinical drug development strategies and its discussions with regulatory agencies.
Cell Specificity: The company is using its capsid engineering capabilities to identify novel AAV capsids with an overall higher tropism for the heart compared to other organs and selectively to target the two most abundant cell types in the heart: cardiomyocytes and cardiac fibroblasts. The company already has achieved in vivo proof of concept for novel vectors for both cell types.
Library Diversity: The company has screened more than one billion variants from 30 diverse libraries utilizing a range of strategies, including rational modification of surface residues, as well as directed evolution efforts with peptide insertion libraries, chimeric libraries, and libraries based on systematic alteration of variable regions using different parental capsids.
Screening Models: The company has performed its screens in a variety of in vitro, in vivo, and in silico libraries.
Screening Criteria: The company has broad criteria for the selection of novel capsids, including improved tropism for the heart compared to other organs, with a particular interest in de-targeting the liver; improved transduction of specific heart cell types; lower susceptibility to neutralizing antibodies; and comparable manufacturing in both HEK293- and Sf9/rBV-based manufacturing systems.
Heart Specificity: The company has developed cardiac-specific promoters that enable more selective and robust expression in the heart as compared to other organs. During optimization of TN-201, the company developed a cardiomyocyte-specific promoter, TNP-CM1, with improved performance attributes as compared to the standard cTnT promoter. In vitro and in vivo analyses confirmed that TNP-CM1 significantly increased expression of the MYBPC3 gene compared to what can be achieved with the standard cTnT promoter. In addition, in a mouse model the ocmpany observed 1000-fold selectivity of expression in cardiac tissue relative to other tissues, including skeletal muscle, brain and liver.
Cell Specificity: The company has also developed a proprietary combination of regulatory elements that enable more optimal and selective expression in one cell type in the heart compared to others. For its Reprogramming program for cellular regeneration, the company discovered ways to optimize the robust co-expression of two protein-coding genes and one micro-RNA gene delivered within a single AAV in cardiac fibroblasts, which supports higher efficacy in preclinical models. The company also discovered how to use specific micro-RNA binding sites to silence the translation of those same genes in both existing cardiomyocytes, as well as newly created cardiomyocytes, which may provide a safety benefit and reduce the chance for off-target effects.
Tunable Gene Expression: The company has also demonstrated the ability to develop an entire spectrum of novel promoters to titer the expression of genes within cardiomyocytes, by combining various combinations of enhancer elements from different cardiomyocyte selective genes. Through data generated in its DWORF program, more than ten promoters were designed and tested in vitro in human iPSC-CMs, and in vivo in murine models to optimize the expression of the DWORF gene to be higher than what can be achieved with a standard cTnT promoter.
The company has fully integrated and internalized AAV manufacturing capabilities to support its Gene Therapy and Cellular Regeneration platforms. The company’s overall strategy is to have complete ownership of its PD, analytical development, MFG and QC so that it has deep insight into the attributes of its drug substance and drug product.
Vector Core: The company has established vector production to support early research involving both parental and novel AAV capsids at the 50L scale.
Manufacturing Technology Development Center (MTDC): The company has established in-house operations at the 200L scale to support all non-clinical studies, including those involving large animal models, such as pigs and NHPs, under GLP regulations. The company also relies on the MTDC for assay development and technology transfer to its dedicated cGMP facility. The company’s initial production at this scale has been at yields and with full/empty capsid ratios that compare favorably to industry standards.
Genetic Medicines Manufacturing Center: The company’s GMMC is a dedicated cGMP facility for AAV drug product manufacturing and is located in the San Francisco Bay Area.
Intellectual property: The company has in-licensed and internally developed certain manufacturing-related intellectual property to support its programs. The company has filed multiple patent applications covering improvements that will support scale-up of AAV manufacturing for supply of its gene therapy product candidates intended for more prevalent heart disease populations.
Strategy
The key components of the company’s strategy are to focus exclusively on heart disease; develop disease-modifying therapies; discover novel therapies using integrated product platforms; target defined sub-populations of patients most likely to respond to its therapies; internalize and integrate core capabilities to support its innovation; advance a deep and diverse pipeline of therapies; seek partnerships that can expand its reach and accelerate its efforts; and become a fully integrated biopharmaceutical company with commercial capabilities.
Research and Development
The company’s research and development expenses were $94.5 million for the year ended December 31, 2022.
Intellectual Property
TN-201: With regard to TN-201, the company owns two issued U.S. patents covering a recombinant adeno-associated virus (rAAV) virion whose vector genome encodes MYBPC3, two pending non-provisional U.S. patent applications, and twenty six pending foreign patent applications. Any U.S. or foreign patents issued from the pending patent applications are expected to expire in 2041, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account. The pending U.S. non-provisional patent applications disclose various aspects of TN-201, including MYBPC3 gene expression vectors, rAAV virions, rAAV viral genomes, expression cassettes, and cover methods of using such compositions for therapeutic indications.
TN-301: With regard to TN-301, the company owns one issued U.S. patent, one pending non-provisional U.S. patent application and twenty-eight pending foreign patent applications. Any U.S. or foreign patents issued from these pending patent applications are expected to expire in 2040, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account. The pending patent applications cover TN-301 and various analogs. The company also owns two patent families that cover methods of treatment of various diseases and disorders with TN-301 and its analogs, with two pending PCT patent applications and one foreign patent application. Any U.S. or foreign patents issued from national stage filings of these PCT patent applications or the pending foreign patent application are expected to expire in 2042, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees and that national phase entries are timely made based upon the pending PCT applications, and without taking potential patent term extensions or adjustments into account. The company also owns one patent family that covers additional HDAC6i compounds, with one pending non-provisional U.S. patent application and three pending foreign patent applications. Any U.S. or foreign patents issued from these pending patent applications are expected to expire in 2040, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account.
TN-401: With regard to TN-401, the company owns four pending U.S. non-provisional patent applications, one pending PCT patent application and three foreign counterparts of these patent applications from national stage filings. Patents claiming priority to these patent applications, if issued, are expected to expire by 2041, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees and without taking potential patent term extensions or adjustments into account. These patent applications are related to proprietary PKP2 gene expression vectors and methods of use. The company owns one pending U.S. provisional patent application related to PKP2 therapeutic treatment methods. Patents claiming priority to this U.S. provisional patent application, if issued, are expected to expire in 2043, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account. The company owns one pending U.S. provisional patent application related to capsids for PKP2 therapy and methods of use. Patents claiming priority to this U.S. provisional patent application, if issued, are expected to expire in 2043, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account.
DWORF: With regard to its DWORF program, the company exclusively licensed two issued U.S. patents and one pending U.S. patent application from UTSW (the UT Patents). The U.S. patents and the pending U.S. patent application, if issued, are expected to expire in 2037, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees and without taking potential patent term extensions or adjustments into account. The UT Patents cover a polynucleotide encoding a DWORF peptide, a vector comprising the same, and methods of enhancing activity of the SERCA pump in a subject using the same. In addition, the company exclusively licensed from UTSW one pending non-provisional U.S. patent application, and fourteen pending foreign patent applications, covering a rAAV virion whose vector genome encodes DWORF and methods of use. Any U.S. or foreign patents issued from the pending patent applications are expected to expire in 2041, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account. Furthermore, the company owns one pending PCT, one pending non-provisional U.S., and two pending foreign patent applications covering proprietary vectors, including proprietary DWORF vectors, and methods of use. Any U.S. or foreign patents if issued from the pending patent applications or from national stage filings of the PCT, are expected to expire in 2042, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees and that national phase entries are timely made based upon the pending PCT application, and without taking potential patent term extensions or adjustments into account.
Reprogramming: The company owns four patent families directed to product candidates in its Reprogramming program, including one issued U.S. patent, three pending non-provisional U.S. patent application, twenty-one foreign counterparts of these patent applications, and a pending PCT patent application. Any U.S. or foreign patents issued from these pending patent applications, or national stage filings of the PCT patent application, are expected to expire between 2039 and 2042, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees and that national phase entries are timely made based upon the pending PCT application, and without taking potential patent term extensions or adjustments into account. The four patent families cover various aspects of its Reprogramming program, including recombinant enhancers, gene delivery vectors, methods of treating a heart condition, engineered myocardin proteins, vectors encoding engineered myocardins, methods of making and methods of use. Additionally, the company owns a fifth patent family that is directed to AAV-based gene vectors and recombinant capsid proteins for cardiac cell transduction, with one pending non-provisional U.S. patent application and nine pending foreign counterparts of this patent application. Any U.S. or foreign patents issued from these pending patent applications are expected to expire in 2040, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account.
Core Capabilities: Capsid Engineering, Manufacturing and Drug Delivery
Capsid Engineering: In support of its efforts to develop novel heart-tropic AAV capsids, the company owns two patent families directed to AAV-based recombinant capsid proteins, with one pending non-provisional U.S. patent application, twenty five pending foreign patent applications, and two pending provisional U.S. patent applications. Any U.S. or foreign patents based on the pending patent applications, or claiming priority to the provisional U.S. patent applications, if issued, are expected to expire between 2041 and 2043, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account.
Manufacturing: In support of its manufacturing activities, the company owns two patent families with one pending non-provisional U.S. patent application and one pending provisional U.S. patent application. The pending non-provisional U.S. patent application is directed to improved methods of baculovirus expression, and any U.S. patent issued from this patent application is expected to expire in 2041, assuming payment of all appropriate maintenance or other governmental fees, and without taking potential patent term extensions or adjustments into account. The pending provisional U.S. patent application is directed to improved methods of viral particle production, and any U.S. or foreign patents claiming priority to this provisional U.S. patent application, if issued, are expected to expire in 2043, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account.
Drug Delivery: In support of its efforts to identify different ROAs, as well as different infusion- or injection-based catheters to support more targeted delivery and more efficient uptake of therapies based on viral vectors, the company owns one pending provisional U.S. patent application directed to cardiac catheter devices and systems for administration of its product candidates, such as gene delivery vectors. Any U.S. or foreign patents claiming priority to this provisional U.S. patent application, if issued, are expected to expire in 2044, assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, and without taking potential patent term extensions or adjustments into account.
Trade Secrets
Through development of internal manufacturing capabilities for AAV-based gene vectors, the company has secured proprietary know-how and trade secrets related to its most-advanced programs as well as vector technologies widely applicable to potential AAV therapies.
Regulation
The company is subject to the U.K. General Data Protection Regulation (GDPR), which implements the GDPR in the U.K. post- Brexit.
History
Tenaya Therapeutics, Inc. was founded in 2016. The company was incorporated in Delaware in 2016.
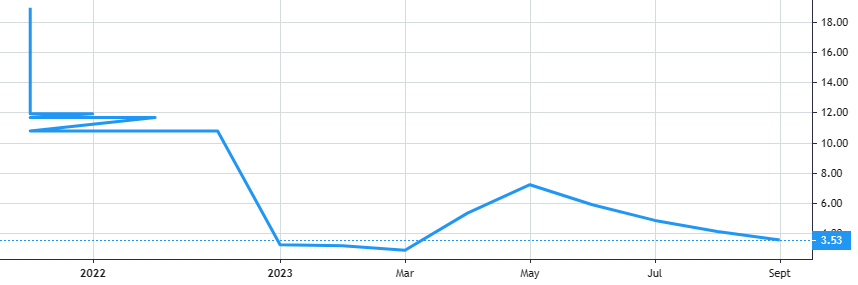
 Stock Value
Stock Value

