About Insulet
Insulet Corporation (Insulet) primarily engages in the development, manufacture and sale of its proprietary continuous insulin delivery systems for people with insulin-dependent diabetes.
The Omnipod platform includes: the Omnipod 5 Automated Insulin Delivery System ('Omnipod 5'), the Omnipod DASH Insulin Management System ('Omnipod DASH'), the Omnipod Insulin Management System ('Classic Omnipod') and the company's latest innovation, Omnipod GO, which received the U.S. Food and Drug Administration ('FDA') clearance in 2023.
The company also produces pods for Amgen for use in the Neulasta Onpro kit, a delivery system for Amgen's Neulasta to help reduce the risk of infection after intense chemotherapy. In the company's international locations the company sells either directly to consumers or through a distributor/intermediary.
Solution: The Omnipod Platform
The Omnipod platform offers continuous insulin delivery that provides all the benefits of insulin pump therapy in a unique way without the need for external tubing required with conventional pumps. The small, lightweight, self-adhesive disposable tubeless Omnipod device ('Pod'), can be worn in multiple locations, including the abdomen, hip, back of upper arm, upper thigh, or lower back, and delivers insulin into the body through a small flexible tube (called a cannula). The company refers to this as 'Pod therapy.' The Omnipod platform's proprietary design and differentiated features allow people with insulin-dependent diabetes to live their lives and manage their diabetes, with unprecedented freedom, comfort, convenience, and ease.
Omnipod 5

Omnipod 5, which builds on the company's Omnipod DASH platform, described below, was cleared by the FDA in January 2022. The company's limited market release of Omnipod 5 in the United States commenced the following month, and in August 2022 the company launched its U.S. full market release. In September 2022, the company received CE Mark approval for Omnipod 5 under the European Union Medical Device Regulation ('MDR') and in 2023, the company launched Omnipod 5 in the United Kingdom and Germany.
Omnipod 5 includes a proprietary AID algorithm embedded in the Pod. The Pod integrates with a third-party continuous glucose monitor ('CGM') to obtain glucose values through wireless Bluetooth communication. The embedded algorithm utilizes these glucose values to predict glucose levels into the future and automatically adjusts insulin dosing intended to improve time-in-range and reduce the occurrence of blood glucose highs and lows. The user can also deliver insulin doses for snacks or meals or to correct high blood glucose through the system. The Pod is controllable by an Insulet-provided handheld device (Controller) or a user-downloaded Android app, which allows for full compatible smartphone control. In addition, in October 2023, the company received FDA clearance for its Omnipod 5 App for iPhone, which allows for control using a compatible iOS smartphone. The company expect to launch the iOS app in 2024. The Omnipod 5 Controller and the Omnipod 5 Android and iOS apps use cloud-based technology to wirelessly upload data using a built-in SIM card for cellular connectivity or from a secure Wi-Fi connection if established. The Pod integrates with Dexcom, Inc.'s G6 CGM and the company recently launched a limited market release with Dexcom's G7 CGM. Additionally, the company plans to launch Omnipod 5 with Abbott Diabetes Care, Inc.'s FreeStyle Libre 2 Plus sensor ('Libre 2 Plus') in certain international markets in 2024.
Omnipod DASH
Omnipod DASH features a secure Bluetooth enabled Pod that is controlled by a smartphone-like Personal Diabetes Manager ('PDM') with a color touch screen user interface. In the U.S., the PDM has Wi-Fi capabilities to enable automatic data uploads providing users and their clinicians with cloud access to data and enhancements for pushing software updates wirelessly to users. Omnipod DASH provides continuous insulin delivery at preset rates, eliminating the need for individual insulin injections. In addition, insulin delivery can be changed with the press of a button to adapt to snacks or unexpected changes in daily routine. Omnipod DASH delivers insulin in two ways:
A small, constant background supply of insulin is delivered automatically at a programmed rate, all day and night.
An extra dose of insulin can be delivered when needed to match the carbohydrates in a snack or meal to correct high blood glucose.
The company has designed Omnipod DASH to fit within the normal daily routines of users. Omnipod DASH communicates wirelessly, provides for virtually pain-free automated cannula insertion, and eliminates the need for MDI therapy or the use of pump and tubing. The Pod can be worn for up to three days at a time and, because it is waterproof (with an IP28 rating for up to 25 feet for 60 minutes), there is no need to remove it when showering, swimming, or performing other activities.
Omnipod Classic
Following the launch of Omnipod 5, the vast majority of the company's customer base is no longer using its Classic Omnipod product. Accordingly, the company is phasing-out its Classic Omnipod product in the U.S. and no longer guarantee the availability of its supplies.
Omnipod GO
Omnipod GO is a standalone, wearable, insulin delivery system that provides a fixed rate of continuous rapid-acting insulin for 72 hours. Omnipod GO has been cleared by the FDA for use by people with type 2 diabetes age 18 and older who would typically take daily injections of long-lasting insulin. The newest addition to the Omnipod brand features a tubeless and waterproof Pod (with an IP28 rating for up to 25 feet for 60 minutes) which is offered in seven different pre-programmed daily rates, ranging from 10 to 40 units per day, and operates without the need for a handheld device to control the Pod. Omnipod GO has been cleared for use with the following U-100 insulins: NovoLog, Fiasp, Humalog, Admelog, and Lyumjev.
The product was developed to serve people with type 2 diabetes earlier in their treatment journey by starting them on Pod therapy for their insulin delivery, rather than daily injections. As a patient progresses to requiring additional insulin, including basal and bolus, the transition from Omnipod GO to another Omnipod product will be a natural progression.
The company developed Omnipod GO with convenience in mind for both the primary care physician and the user, including with respect to prescribing, getting started, training and using the product. The company is conducting a pilot program for Omnipod GO in the United States.
Data Management
The company have partnered with Glooko Inc. ('Glooko') to connect user data with Glooko's comprehensive diabetes data management system (including Glooko and Diasend in selected regions). Glooko provides a cloud-based application for clinicians and users accessible through a kiosk, home computer, or a mobile application on the user's smartphone that provides users and their healthcare providers access to insulin delivery trends, blood glucose levels, and other integrated data.
Security
Paramount to the company's ability to deliver full compatible smartphone control is the company's commitment to cybersecurity and information security. With certifications from the Diabetes Technology Society's 'Standard for Wireless Diabetes Device Security' cybersecurity and assurance standard and program as well as from the International Organization for Standardization ('IOS'), Insulet is globally recognized for incorporating the highest standards for cybersecurity, information security and safety, including secure data transfer between the Pod and PDM, as well as secure cloud storage.
In October 2023, the company received FDA clearance for an iOS app that will enable control of Omnipod 5 Pods using an iPhone and plan to launch a limited market release in the U.S. in 2024. Further, the company recently launched a limited market release for Omnipod 5 integration with Dexcom's G7 CGM in the United States. The company also recently received CE mark approval under the MDR for the added compatibility of Libre 2 Plus with Omnipod 5 for individuals aged two years and older with type 1 diabetes and expect to launch a limited market release of Omnipod 5 with Libre 2 Plus in the U.K. and the Netherlands in 2024.
The company also continues to advance work to improve the Omnipod 5 algorithm and develop next-generation automated insulin delivery products. In 2023, the company began its EVOLUTION feasibility trial in New Zealand to test potential enhancements to the Omnipod 5 algorithm in order to further drive simplicity of use. The company has completed the first round of study, which included testing the system with both type 1 and type 2 users and are in the process of analyzing the data and making modifications for the next round of study.
In December 2023, the company completed enrollment in its pivotal trial for Omnipod 5 with the goal of expanding its indication to individuals with type 2 diabetes. The company expects to complete the trial and submit the company's 510(k) application to the FDA by the end of 2024. Further, in April 2023, the company received FDA clearance for Omnipod GO, a basal-only Pod for individuals with type 2 diabetes.
Markets and Distribution Methods
Omnipod products are available in the following 25 countries: Australia, Cyprus, Greece, Netherlands, Switzerland, Austria, Denmark, Iceland, Norway, Turkey, Belgium, Finland, Israel, Qatar, the United Arab Emirates, Canada, France, Italy, Saudi Arabia, the United Kingdom, Croatia, Germany, Kuwait, Sweden, and the United States.
The company sells Omnipod products directly to consumers, through distribution partners and in the U.S., also through the pharmacy channel. For the year ended December 31, 2023, 90% of Omnipod product sales globally were through intermediaries.
The company's sales and marketing efforts are focused on customer acquisition and retention to meet the user, clinician, and payor demands for the company's Omnipod products. The company has a comprehensive sales and marketing approach, which communicates the benefits of the Omnipod platform to users, physicians and providers. This includes three areas of focus:
Building consumer awareness about the features and benefits that Omnipod products provide to simplify diabetes management.
Strengthening physician support by demonstrating clinical evidence of how Omnipod products improve outcomes and quality of life and providing data and insights to physicians offering diabetes care.
Providing payors with the clinical and economic justifications for why Omnipod products offer unique value to the people they insure.
Competition
The company competes with companies in the insulin pump market, which consists of tubed pump companies, including Medtronic MiniMed, a division of Medtronic public limited company ('Medtronic'), and Tandem Diabetes Care Inc. ('Tandem').
Intellectual Property
Patents
As of December 31, 2023, the company had over 700 patents in the United States and in certain other countries, with expiration dates ranging from 2024 through 2043 and had over 500 patent applications pending. The issued patents and pending patent applications cover, among other things: the basic architecture of the company's Omnipod products, including the pump and the PDM/Controller; the Omnipod drive system; the Omnipod cannula insertion system; software, such as algorithms and apps, for controlling the company's current and next generation Omnipod products; and various novel aspects of the company's future generations of Omnipod products, and other mechanisms for the delivery of pharmaceuticals.
Trademarks
The company has registered various trademarks associated with its business with the United States Patent and Trademark Office on the Principal Register and in other appropriate jurisdictions. The company's trademarks include INSULET, OMNIPOD, SIMPLIFY LIFE, Omnipod DASH, Omnipod GOTM, Omnipod DISPLAY, Omnipod VIEW, SmartAdjustTM, Pod Pals, Podder, and PodderCentral.
Government Regulation
The company's products are medical devices that are subject to extensive and ongoing regulation by the FDA and other federal, state, and local regulatory bodies.
The company has obtained 510(k) clearance for Classic Omnipod, Omnipod DASH, Omnipod 5, and Omnipod GO.
The company is subject to the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary's selection of a particular supplier of Medicare or Medicaid payable items or services.
Many states have adopted anti-kickback, anti-referral laws, and false claims laws and regulations analogous to the federal civil Anti-Kickback Statute and federal False Claims Act. In some cases, these state laws apply regardless of the payor, including private payors. The company is in compliance with such laws.
The company is subject to FCPA in the U.S. and to similar anti-bribery laws in other jurisdictions, which generally prohibit companies and their intermediaries from making improper payments to foreign officials for the purpose of obtaining or retaining business.
The company has obtained the right to affix the CE Mark to Classic Omnipod and Omnipod DASH under the MDD. The company has obtained the right to affix the CE Mark to Omnipod 5 under MDR, which allows the company to distribute it throughout the European Union and in the United Kingdom. In addition to these countries, Omnipod DASH can be marketed in the Gulf Cooperation Council Countries and in Australia. The company also has Health Canada approval to sell Classic Omnipod and Omnipod DASH in Canada.
A range of anti-bribery and anti-corruption laws, as well as industry specific laws and codes of conduct, apply to the medical device industry and interactions with government officials and entities and healthcare professionals. These laws include the U.K. Bribery Act and similar antibribery laws in other jurisdictions in which the company operates.
History
Insulet Corporation, a Delaware corporation, was founded in 2000. The company was incorporated in 2000.
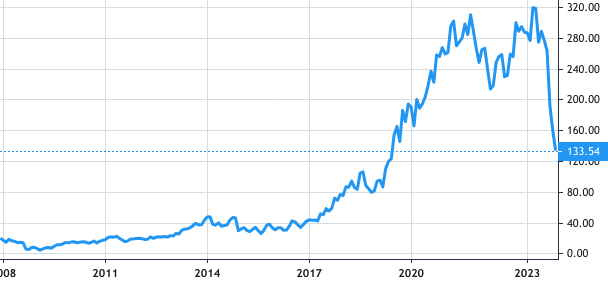
 Stock Value
Stock Value

