About Moderna
Moderna, Inc. operates as a biotechnology company. The company is advancing a new class of medicines made of messenger RNA (mRNA). mRNA medicines are designed to direct the body's cells to produce intracellular, membrane or secreted proteins that have a therapeutic or preventive benefit with the potential to address a broad spectrum of diseases.
The company's platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, providing it the capability to pursue in parallel a robust pipeline of new development candidates. The company is developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases, independently and with its strategic collaborators.
The company is a leader in the creation of the field of messenger RNA (mRNA) medicine. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
The company's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. The company's first commercial product, Spikevax (COVID-19 vaccine), has helped various people worldwide combat COVID-19. To adapt to the evolving market, the company significantly resized its manufacturing infrastructure to help position its COVID-19 franchise for future profitability.
Beyond COVID-19, in 2023, the company prepared for the potential 2024 launch of its investigational respiratory syncytial virus (RSV) vaccine for adults, which it expects to further demonstrate the commercial potential of its mRNA platform. In cancer, the company reported additional data from its Phase 2b trial evaluating its individualized neoantigen therapy (INT) in combination with Merck's KEYTRUDA in melanoma patients compared to KEYTRUDA alone. The company and Merck plan to rapidly expand its clinical trials to additional tumor types. The company's pipeline includes 45 therapeutic and vaccine programs, nine of which are in late-stage development.
Strategy

The key elements of the company's strategy are to deliver an unrivalled respiratory vaccine franchise; advance multiple latent virus and other vaccines; accelerate a large portfolio of late-stage clinical trials in INT to deliver a transformative impact in cancer treatments; accelerate investment in three rare disease programs to pursue potential launches; and deliver the next-generation pipeline and platform.
Platform
The company's mRNA platform refers to its accumulated knowledge and capabilities in basic and applied sciences. The company's platform incorporates advances across three key components-mRNA, delivery and the manufacturing process- to advance its medicines. The company integrates these components and combine different versions of mRNA delivery and process into each of its medicines.
Platform: mRNA Science Advancements
The company continues to invest in both basic and applied research, seeking to advance both the state of its technology and the state of the scientific community's understanding of mRNA.
The company's platform applies bioinformatic, biochemical, and biological screening capabilities, most of which have been invented internally that aim to optimize the amount of protein produced per mRNA. The company has identified proprietary sequences for the 5'-UTR that have been observed to increase the likelihood that a ribosome bound to the 5'-end of the mRNA transcript will find the desired start codon and reliably initiate translation of the coding region. The company additionally designs the nucleotide sequence of the coding region to maximize its successful translation into protein. The company designs microRNA binding sites into the 3'-UTR of its potential mRNA medicines so that if its mRNA is delivered to cells with such microRNAs, it will be minimally translated and rapidly degraded.
Commercial
Before 2023, the company sold its COVID-19 vaccine to the U.S. Government, foreign governments and health ministries, Gavi, on behalf of the COVAX Facility, and other international organizations. During the pandemic, these sales were characterized by a relatively limited number of customers that purchased multi-dose vials for distribution through mass vaccination campaigns. As the company has transitioned to an endemic market, it has witnessed a shift in demand to single-dose presentations and pre-filled syringes.
Third-Party Strategic Alliances
Strategic Alliances
Representative relationships and associated programs include those with Merck, for the company's INT programs (mRNA-4157), and Vertex, for its CF program (mRNA-3692).
In 2023, the company's Strategic Collaboration and License Agreement with Vertex (the Vertex 2020 Agreement) was concluded and terminated in accordance with the terms of the agreement. The Vertex 2020 Agreement had been aimed at the discovery and development of potential medicines to treat cystic fibrosis (CF) with gene-editing therapies.
Merck-Strategic Alliance for Individualized Neoantigen Therapies
In 2016, the company entered into a Collaboration and License Agreement with Merck for the development and commercialization of personalized mRNA cancer vaccines (also known as INTs), which was subsequently amended and restated in 2018 (the INT Agreement), to develop and commercialize INTs for individual patients using its mRNA vaccine and formulation technology. Under the strategic alliance, the company identifies genetic mutations present in a particular patient's tumor cells, synthesize mRNA for these mutations, encapsulate the mRNA in one of its proprietary LNPs and administer to each patient a unique INT designed to specifically activate the patient's immune system against own cancer cells.
Vertex-2016 Strategic Alliance in Cystic Fibrosis
In 2016, the company entered into a Strategic Collaboration and License Agreement (Vertex Agreement) with Vertex Pharmaceuticals Incorporated, and Vertex Pharmaceuticals (Europe) Limited (together, Vertex). The Vertex Agreement is aimed at the discovery and development of potential mRNA medicines for the treatment of CF by enabling cells in the lungs of people with CF to produce functional cystic fibrosis transmembrane conductance regulator (CFTR) proteins.
Other Collaborations
In September 2020, the company entered into a collaboration with Chiesi Farmaceutici S.p.A. (Chiesi), an international research-focused healthcare group, aimed at the discovery and development of mRNA medicines for the treatment of pulmonary arterial hypertension (PAH), a rare disease characterized by high blood pressure in the arteries of the lungs.
Defense Advanced Research Projects Agency (DARPA)
In 2020, the company entered into an agreement with DARPA to fund development of a mobile manufacturing prototype leveraging its existing manufacturing technology that is capable of rapidly producing vaccines and therapeutics.
Institute for Life Changing Medicines (ILCM)
In 2021, the company entered into a collaboration agreement with the ILCM to develop a new mRNA therapeutic (mRNA-3351) for type 1 Crigler-Najjar syndrome (CN-1). Under the terms of the agreement, it agreeds to license mRNA-3351 to ILCM with no upfront fees, and without any downstream payments. ILCM will be responsible for the clinical development of mRNA-3351.
The Bill & Melinda Gates Foundation
In 2016, the company entered a global health project framework agreement with the Bill & Melinda Gates Foundation to advance mRNA development projects for various infectious diseases.
Intellectual Property
The company has built a substantial IP estate that includes numerous patents and patent applications related to the development and commercialization of mRNA vaccine and therapeutic development candidates, including related platform technologies. The company's platform IP protects advances in mRNA design and engineering, proprietary LNP components, delivery systems, processes for the manufacture and purification of drug substances and products and analytical methods. A significant portion of the company's platform IP estate further provides multi-layered protection for its modalities and programs.
With respect to its IP estate, the company's solely-owned patent portfolio consists of more than 230 issued or allowed U.S. patents or patent applications and more than 170 granted or allowed patents in jurisdictions outside of the U.S. (including granted European patents that have been validated in numerous European countries) covering certain of its proprietary platform technology, inventions and improvements, and covering key aspects of its clinical and most advanced development candidates. The company has over 400 additional pending patent applications that, in many cases, are counterparts to the foregoing U.S. and foreign patents.
Most of the patents and applications (if issued) in the company's portfolio will not expire until 2033 at the earliest. Any patent that may issue from the company's most recently filed patent applications is projected to expire between 2042 and 2043, at the earliest. The company files additional U.S. and foreign patent applications as necessary to protect its evolving intellectual property positions.
IP Protecting Platform
The company has a broad IP estate covering key aspects of its platform. This estate provides multiple layers of protection covering the making and use of the mRNA drug substance and delivery technologies.
With respect to its platform, the company has a portfolio that includes U.S. and foreign patents or patent applications covering platform innovations that are related to the design, manufacturing and formulating of mRNA medicines.
IP Protection
The company's IP estate provides protection for the multiple programs both at the product-specific level and at various broader levels. For example, the company has patent coverage for LNP-encapsulated mRNAs having specific chemical modification suited for vaccine and therapeutic mRNA use. The company's estate also includes IP covering certain LNP-encapsulated mRNAs coding for infectious disease antigens for use in preventing or treating infectious diseases, including those caused by respiratory and latent viruses, as well as bacterial, viral and parasitic diseases known to threaten public health.
Respiratory Vaccines
For its respiratory vaccines programs, the company has pursued patent protection featuring composition of matter and method of use claims. The company has filed several patent applications covering its betacoronavirus vaccine program. The company is pursuing patent protection for both its existing and next generation betacoronavirus vaccines.
RSV
The company has filed multiple patent families directed to RSV vaccines, including a U.S. patent that issued on October 11, 2022. The company's RSV patent portfolio includes multiple families of differing patent breadth. At least four U.S. and European patent applications are pending.
Influenza
The company has multiple patent families spanning different levels of breadth, design and antigen valency pending in the U.S., Europe and around the world, including several granted patents.
hMPV
Human metapneumovirus (hMPV) is a single-stranded RNA virus that is used in a combination program. The company has patent applications covering its hMPV vaccine pending in the U.S. and Europe, with a granted patent in the U.S.
Latent Vaccines
The company has vaccine programs and patent applications directed to both the acute and latent forms of diseases caused by various viruses, including CMV, EBV, HSV, VZV and HIV, using both preventative vaccines targeting the acute phase and therapeutic vaccines for treating the latent diseases in those who do become infected.
CMV
The patent coverage for the company's human CMV vaccine candidate is extensive and is based on a vaccine with six mRNAs encoding a pentamer surface glycoprotein complex and the gB surface glycoprotein. Both pentamer and gB facilitate entry of the virus into different cell types and therefore immune responses targeting these proteins can block virus entry, spread and reactivation. The current patent portfolio contains both compositions of matter and methods of treating subjects using the vaccine. In the U.S., the company's CMV vaccine is covered by multiple issued U.S. patents of differing breadth. Each family has counterparts consisting of pending applications and issued patents in non-U.S. jurisdictions, including Europe and Japan. A separate family of CMV patents, which includes mRNA-1647 plus mRNA-1443 for use in CMV vaccines for transplant indications, is also yielding patents and applications in foreign jurisdictions are pending.
EBV, HSV and VZV
Similar to CMV, the company has multiple patent families pending for each of EBV, HSV and VZV, covering both prophylactic and therapeutic indications. These patent families have also been filed in Europe and Japan.
Public Health Vaccines
The company maintains a multi-program effort at developing vaccines for potential future pandemics and for use in parts of the world with less well-established health care systems. This group of programs include infectious diseases such as flaviviruses such as Zika and dengue viruses, HIV, Nipah virus, and the Mpox virus. In addition, programs are ongoing in many bacterial diseases.
Individualized Neoantigen Therapy (INT)
Composition of matter and method claims are being pursued to protect programs within the company's cancer vaccines modality. Proprietary methods around the making and therapeutic use of the company's INTs and resulting vaccine compositions are described and claimed in seven pending U.S. patent applications, six pending European patent applications, five pending patent applications in Japan, three pending patent applications and one granted patent in China, and several pending patent applications in New Zealand, South Africa, Asian and South American countries, as well as one PCT application. The company also possesses substantial know-how and trade secrets relating to the development and commercialization of its cancer vaccine programs, including related manufacturing process and technology.
Likewise, the company's KRAS antigen cancer vaccine and methods of treating cancer featuring such vaccines are covered in an issued U.S. patent, which includes claims to LNP-encapsulated mRNA encoding mutant KRAS antigens.
Intratumoral Immuno-Oncology
The company has filed numerous patent applications featuring claims to mRNAs encoding immune-stimulatory proteins and methods of treating cancer using such compositions.
The company's immuno-oncology programs are designed to be administered intratumorally to alter the tumor microenvironment in favor of mounting an immune response against tumors. The company's mRNA program that includes mRNAs that encode OX40L, IL-23 and IL-36gamma are covered by two granted European patents, by more than 10 issued U.S. patents, by several pending U.S. and European patent applications and by several pending patent applications in other foreign jurisdictions.
Rare Diseases
The company has programs featuring expression of therapeutic proteins, e.g., intracellular enzymes for the treatment of rare diseases. For its rare disease programs, the company generally pursues patent protection featuring composition of matter and method of use claims, for example, pharmaceutical composition and method of treatment claims. The company's most advanced rare disease development candidate is for PA. For this candidate, the company has patent applications pending in the United States, Europe, and Japan which cover mRNA encoding the alpha and beta subunits of the enzyme propionyl-CoA carboxylase (PCCA and PCCB, respectively), for the treatment of PA.
For MMA, the company has patent applications issued and pending in the U.S. and foreign applications filed in Japan and Europe.
For its PKU development candidate, the company has a pending PCT and pending patent applications in the U.S., Europe and Japan covering mRNA encoding phenylalanine hydroxylase (PAH) for the treatment of PKU.
For its Glycogen Storage Disorder, Type 1a (GSD1a) development candidate, the company has filed several patent families, including pending U.S. and European patent applications, as well as applications pending in China and Japan covering mRNA encoding glucose 6-phosphatase (G6Pase) for the treatment of this disorder.
For its Crigler-Najjar Syndrome Type 1 (CN-1) development candidate, the company has patent applications pending in the U.S., Europe and Japan.
The company's ornithine transcarbamylase deficiency (OTC) development candidate is covered by a pending PCT application.
Any U.S. and foreign patents that may issue from these patent families would be expected to expire in 2036 for the earliest of the MMA patents and 2038 to 2042 for the remaining MMA, PA, PKU, GSD1a and CN-1 patents, excluding any patent term adjustments, any patent term extensions and any terminal disclaimers.
The company has filed or intend to file patent applications on these and other aspects of its technology and development candidates, and as it continues the development of its intended products, it plans to identify additional means of obtaining patent protection that would potentially enhance commercial success, including protection for additional methods of use, formulation, or manufacture.
Systemic Secreted and Cell-Surface Therapeutics
The company's systemic secreted and cell-surface therapeutics modality features programs directed to expression of secreted or cell-surface proteins including antibodies, circulating immune modulation factors, secreted enzymes and transmembrane proteins.
The company's Relaxin development candidate is covered by several patent families, including granted patents in Japan, China and the U.S., and by additional applications in the U.S. and additional foreign jurisdictions and a pending PCT application. The company's PD-L1 development candidate is covered in pending patent applications filed in the U.S., Japan and Europe.
Inhaled Pulmonary Therapeutics
The company's inhaled pulmonary therapeutics modality has one development candidate directed to expression of therapeutic protein in the lungs. This Cystic Fibrosis (CF) development candidate is covered by pending U.S., European and PCT patent applications.
Gene Editing
The company's gene editing program has one filed patent family that includes issued patents in the U.S., Europe and Japan, and also pending applications in these jurisdictions. The company plans to file patent applications on development candidates and other aspects of gene editing technology as it continues to innovate both internally and through strategic collaborations.
Trademarks
The company's trademark portfolio contains at least 1,000 trademark registrations, including at least 22 registrations in the United States and the remaining in Canada, the European Union, the United Kingdom, Israel, China, Japan, Australia, and elsewhere. In addition, the company has at least 600 pending trademark applications in more than 95 jurisdictions, including in the aforementioned locations and additional countries throughout Africa, Asia, and South America.
In-Licensed Intellectual Property
While it develops and manufactures its potential mRNA medicines using its internally created mRNA technology platform, the company also seeks out and evaluate third party technologies and IP that may be complementary to its platform.
Patent Sublicense Agreements with Cellscript and mRNA RiboTherapeutics
The Trustees of the University of Pennsylvania owns several issued U.S. patents, granted European patents and pending U.S. patent applications directed, in part, to nucleoside-modified mRNAs and their uses (the Penn Modified mRNA Patents). mRNA RiboTherapeutics, Inc. (MRT) obtained an exclusive license to the Penn Modified mRNA Patents and granted its affiliate, Cellscript, LLC (Cellscript), a sublicense to the Penn Modified mRNA Patents in certain fields of use.
In June 2017, the company entered into two sublicense agreements, one with Cellscript, and one with MRT, which agreements it collectively refers to as the Cellscript-MRT Agreements. Together, the Cellscript-MRT Agreements grant the company a worldwide, sublicensable sublicense to the Penn Modified mRNA Patents to research, develop, make, and commercialize products covered by the Penn Modified mRNA Patents (licensed products), for all in vivo uses in humans and animals, including therapeutic, prophylactic, and diagnostic applications. The Cellscript-MRT Agreements are non-exclusive, although Cellscript and MRT are subject to certain time restrictions on granting additional sublicenses for in vivo uses in humans under the Penn Modified mRNA Patents.
Patent License Agreement with NIAID
In December 2022, the company entered into a non-exclusive patent license agreement with the National Institute of Allergy and Infectious Diseases (NIAID), an Institute or Center of the National Institutes of Health (NIH) to license certain patent rights concerning stabilizing prefusion coronavirus spike proteins and the resulting stabilized proteins for use in COVID-19 vaccine products.
Competition
The company's COVID-19 vaccine largely competes against Pfizer/BioNTech's COVID-19 vaccine, which is also based on mRNA technology. The company also competes against other approved or authorized products, including Novavax's COVID-19 vaccine.
Government Regulation
The company is subject to state and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservation and Recovery Act and the Toxic Substances Control Act.
History
The company was founded in 2010. It was incorporated under the laws of the state of Delaware in 2016. The company was formerly known as Moderna Therapeutics, Inc. and changed its name to Moderna, Inc. in 2018.

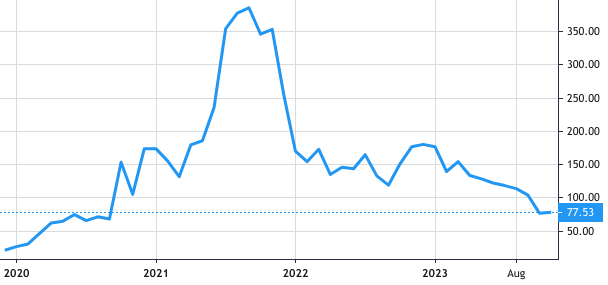
 Stock Value
Stock Value

