About Lexicon Pharmaceuticals
Lexicon Pharmaceuticals, Inc. operates as a biopharmaceutical company.
The company is devoting most of its resources to the research, development and preparation for commercialization of the company’s most advanced drug candidates:
The company has a pending New Drug Application, or NDA, for sotagliflozin, an orally-delivered small molecule drug candidate, as a treatment for heart failure. The NDA is under review by the U.S. Food and Drug Administration, or FDA, and has been assigned a target action date of May 27, 2023 under the Prescription Drug User Fee Act VI, or PDUFA. The NDA is supported by positive results from two Phase 3 clinical trials evaluating the effect of sotagliflozin on long-term outcomes related to cardiovascular death and heart failure in approximately 10,500 and 1,200 patients, respectively. Under the NDA, the company is seeking regulatory approval to market sotagliflozin to reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure and in adults with type 2 diabetes mellitus, chronic kidney disease and other cardiovascular risk factors. The company is preparing for the anticipated commercial launch of sotagliflozin in the United States following approval.
The company has also engaged in the development of sotagliflozin in type 1 diabetes, which was the subject of a separate NDA. That NDA was supported by positive results from three Phase 3 clinical trials evaluating the effect of sotagliflozin on type 1 diabetes in approximately 800, 800 and 1,400 patients, respectively. The FDA issued a complete response letter regarding the company’s NDA for sotagliflozin in type 1 diabetes. At the company’s request, the FDA has issued a public Notice of Opportunity for Hearing on whether there are grounds for denying approval of the company’s NDA and the hearing process is ongoing.
The company is developing LX9211, an orally-delivered small molecule drug candidate, as a treatment for neuropathic pain. The company has reported positive results from a Phase 2 clinical trial of LX9211 in diabetic peripheral neuropathic pain. The company has reported top-line results from a separate Phase 2 clinical trial of LX9211 in post-herpetic neuralgia which demonstrated clear evidence of effect. LX9211 has received Fast Track designation from the FDA for development in diabetic peripheral neuropathic pain.
The company is conducting preclinical research and development and preparing to conduct clinical development of compounds from a number of additional drug programs originating from the company’s internal drug discovery efforts.

Sotagliflozin and compounds from a number of additional drug programs originated from the company’s own internal drug discovery efforts, and LX9211 originated from the company’s collaborative neuroscience drug discovery efforts with Bristol-Myers Squibb. The company’s efforts were driven by a systematic, target biology-driven approach in which the company used gene knockout technologies and an integrated platform of advanced medical technologies to systematically study the physiological and behavioral functions of almost 5,000 genes in mice and assessed the utility of the proteins encoded by the corresponding human genes as potential drug targets. The company has identified and validated in living animals, or in vivo, more than 100 targets with promising profiles for drug discovery.
The company is working both independently and through collaborations and strategic alliances with third parties to capitalize on the company’s drug target discoveries and drug discovery and development programs. The company seeks to retain exclusive or co-exclusive rights to the benefits of certain drug discovery and development programs by developing and commercializing drug candidates from those programs internally, particularly in the United States for indications treated by specialist physicians. The company seeks to collaborate with other pharmaceutical and biotechnology companies with respect to drug discovery or the development and commercialization of certain of the company’s drug candidates, particularly with respect to commercialization in territories outside the United States or commercialization in the United States for indications treated by primary care physicians, or when the collaboration may otherwise provide the company with access to expertise and resources that the company does not possess internally or are complementary to the company’s own.
Drug Development Programs
The company is devoting most of its resources to the research, development and preparation for commercialization of the company’s most advanced drug candidates: sotagliflozin, which the company is developing as a treatment for heart failure and type 1 diabetes, and LX9211, which the company is developing as a treatment for neuropathic pain. The company has also advanced a number of additional compounds into various stages of clinical and preclinical development.
Sotagliflozin
Sotagliflozin is an orally-delivered small molecule compound for which the company has a pending NDA for heart failure and that the company has separately been developing for type 1 diabetes. The company’s scientists identified the targets of sotagliflozin, sodium-glucose cotransporter type 1, or SGLT1, and sodium-glucose cotransporter type 2, or SGLT2, in the company’s target discovery efforts based on their discovery that mice lacking SGLT1, SGLT2 or both exhibited favorable phenotypes across multiple measures of glucose control and metabolism in preclinical models. Preclinical studies of sotagliflozin demonstrated that compounds inhibiting both targets had a favorable preclinical profile relative to compounds selective for SGLT2.
Heart Failure
The FDA is reviewing the company’s NDA for sotagliflozin in heart failure and has assigned a PDUFA target action date of May 27, 2023. Under the NDA, the company is seeking regulatory approval to market sotagliflozin to reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure and in adults with type 2 diabetes mellitus, chronic kidney disease and other cardiovascular risk factors.
The company has completed two Phase 3 clinical trials evaluating the safety and tolerability of sotagliflozin and its effects on long-term outcomes related to composite primary endpoints of total cardiovascular death, hospitalizations for heart failure and urgent visits for heart failure.
The company’s SCORED Phase 3 clinical trial enrolled 10,584 patients with type 2 diabetes, chronic kidney disease with an estimated glomerular filtration rate, or eGFR, of 25 to 60 ml per minute per 1.73 m2 of body surface area and risks for cardiovascular disease in a randomized, double-blind, placebo-controlled study of sotagliflozin added to standard of care over a median treatment period of 16 months.
The company’s SOLOIST Phase 3 clinical trial enrolled 1,222 patients with type 2 diabetes who had recently been hospitalized with worsening heart failure in a randomized, double-blind, placebo-controlled study of sotagliflozin initiated either before or within three days of hospital discharge over a median treatment period of nine months.
Results from both the SCORED and SOLOIST clinical trials were published in the New England Journal of Medicine in November 2020.
Type 1 Diabetes
The company previously filed an NDA for sotagliflozin in type 1 diabetes, regarding which the FDA issued a complete response letter in March 2019 and confirmed that position in denying two appeals of the complete response letter in November 2019 and March 2020. In November 2020, the company requested an opportunity for an administrative hearing on whether there are grounds for denying approval of the company’s NDA. In response to such request, the FDA issued a public Notice of Opportunity for Hearing in March 2021 and the hearing process is ongoing.
In January 2021, sotagliflozin was approved in the United Kingdom for use as an adjunct to insulin therapy to improve glycemic control in adults with type 1 diabetes and a body mass index > 27 kg/m2 , who could not achieve adequate glycemic control despite optimal insulin therapy. The company has not commercially launched sotagliflozin for the treatment of type 1 diabetes in the United Kingdom or any other region.
The company has completed three Phase 3 clinical trials evaluating the safety and tolerability of sotagliflozin and its effects on glycemic parameters associated with type 1 diabetes.
The company’s pivotal inTandem1 Phase 3 clinical trial enrolled 793 patients with type 1 diabetes in the United States and Canada in a randomized, double-blind, placebo-controlled study of 200mg and 400mg once daily doses of sotagliflozin over a 24-week treatment period, followed by a 28-week extension.
The company’s pivotal inTandem2 Phase 3 clinical trial enrolled 782 patients with type 1 diabetes in Europe and Israel in a randomized, double-blind, placebo-controlled study of 200mg and 400mg once daily doses of sotagliflozin over a 24-week treatment period, followed by a 28-week extension.
The company has additionally reported pooled continuous glucose monitoring, or CGM, data from the inTandem1 and inTandem2 clinical trials.
The company’s inTandem3 Phase 3 clinical trial enrolled 1,405 patients with type 1 diabetes in the United States and Europe in a randomized, double-blind, placebo-controlled study of a 400mg once daily dose of sotagliflozin over a 24-week treatment period. Insulin therapy was not optimized in patients and eligibility criteria included any background insulin therapy. Results from the inTandem3 trial were published in the New England Journal of Medicine in September 2017.
LX9211
LX9211 is an orally-delivered small molecule compound that the company is developing as a treatment for neuropathic pain. The company’s scientists identified the target of LX9211, adapter-associated kinase 1, or AAK1, in the company’s target discovery efforts based on their discovery that mice lacking AAK1 exhibited increased resistance to induced neuropathic pain in preclinical models. LX9211 and another development candidate were discovered by scientists working within the company’s drug discovery alliance with Bristol-Myers Squibb from which the company holds exclusive development and commercialization rights. Preclinical studies of LX9211 demonstrated central nervous system penetration and reduction in pain behavior in models of neuropathic pain without affecting opiate pathways. LX9211 has received Fast Track designation from the FDA for development in diabetic peripheral neuropathic pain, or DPN.
The company has completed two Phase 2 clinical trials evaluating LX9211 in neuropathic pain. The first trial, RELIEF-DPN-1, evaluated the safety and tolerability of LX9211 and its effects on DPN, and the second trial, RELIEF-PHN-1, evaluated the safety and tolerability of LX9211 and its effects on post-herpetic neuralgia, or PHN.
The company’s RELIEF-DPN-1 clinical trial enrolled 319 patients experiencing DPN in a randomized, double-blind, placebo-controlled study of LX9211 evaluating three treatment groups receiving an initial loading dose of 100mg or 200mg of LX9211 or placebo, followed by once daily doses of 10mg or 20mg of LX9211 or placebo, respectively.
The company’s RELIEF-PHN-1 clinical trial enrolled 79 patients experiencing PHN in a randomized, double-blind, placebo-controlled study of LX9211 evaluating two treatment groups receiving an initial loading dose of 200mg of LX9211 or placebo, followed by once daily doses of 20mg of LX9211 or placebo, respectively.
Additional Drug Discovery and Development Programs
The company is conducting preclinical research and development and preparing to conduct clinical development of compounds from a number of additional drug programs originating from the company’s internal drug discovery efforts. Those efforts were driven by a systematic, target biology-driven approach in which the company used gene knockout technologies and an integrated platform of advanced medical technologies to systematically study the physiological and behavioral functions of almost 5,000 genes in mice and assessed the utility of the proteins encoded by the corresponding human genes as potential drug targets. The company has identified and validated in living animals, or in vivo, more than 100 targets with promising profiles for drug discovery.
Collaborations and Strategic Alliances
The company is working both independently and through collaborations and strategic alliances with third parties to capitalize on the company’s drug target discoveries and drug discovery and development programs.
Bristol-Myers Squibb
The company established a drug discovery alliance with Bristol-Myers Squibb Company in December 2003 to discover, develop and commercialize small molecule drugs in the neuroscience field. Bristol-Myers Squibb extended the target discovery term of the alliance in May 2006. The company initiated the alliance with a number of neuroscience drug discovery programs at various stages of development and used the company’s gene knockout technologies to identify additional drug targets with promise in the neuroscience field. For those targets that were selected for the alliance, the company and Bristol-Myers Squibb worked together, on an exclusive basis, to identify, characterize and carry out the preclinical development of small molecule drugs. Bristol-Myers Squibb has the first option to assume full responsibility for clinical development and commercialization of any drugs resulting from the alliance, which enter clinical trials, other than LX9211 and additional compounds acting through AAK1, for which the company holds exclusive development and commercialization rights under the alliance.
Genentech
The company established a drug discovery alliance with Genentech, Inc. in December 2002 to discover novel therapeutic proteins and antibody targets. The company and Genentech expanded the alliance in November 2005 for the advanced research, development and commercialization of new biotherapeutic drugs. Under the original alliance agreement, the company used its target validation technologies to discover the functions of secreted proteins and potential antibody targets identified through Genentech’s internal drug discovery research. In the expanded alliance, the company conducted additional, advanced research on a broad subset of those proteins and targets. The company has exclusive rights to develop and commercialize biotherapeutic drugs for two of these targets, while Genentech has exclusive rights to develop and commercialize biotherapeutic drugs for the other targets. The company retains certain other rights to discoveries made in the alliance, including non-exclusive rights, along with Genentech, for the development and commercialization of small molecule drugs addressing the targets included in the alliance.
Other Collaborations
The company has established collaborations with a number of pharmaceutical and biotechnology companies, research institutes and academic institutions under which the company has received fees in exchange for generating knockout mice for genes requested by the collaborator, providing phenotypic data with respect to such knockout mice or otherwise granting access to some of the company’s technologies and discoveries.
Government Regulation
Products manufactured or distributed by the company pursuant to FDA approvals are subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon the company and its third-party manufacturers.
In addition, most healthcare providers who are expected to prescribe the company’s products and from whom the company obtains patient health information, are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology and Clinical Health Act, or HIPAA.
For those marketed products, which are covered in the United States by the Medicaid program, the company has various obligations, including government price reporting and rebate requirements, which generally require products be offered at substantial rebates/discounts to Medicaid and certain purchasers. The company is also required to discount such products to authorized users of the Federal Supply Schedule of the General Services Administration, under which additional laws and requirements apply.
In addition to the foregoing, the company’s business is subject to regulation under various state and federal environmental and worker safety laws, including the Occupational Safety and Health Act; the Resource Conservation and Recovery Act; the Comprehensive Environmental Response, Compensation and Liability Act; and the Toxic Substances Control Act, each as amended from time to time.
Patents and Proprietary Rights
The company owns or exclusively licenses patents and patent applications throughout the world that claim the company’s products and drug candidates, including:
Issued patents and pending patent applications in Europe, the United States, and other countries throughout the world, including Australia, Argentina, Brazil, Canada, China, Europe, India, Israel, Japan, Mexico, New Zealand, South Africa, and South Korea, that claim sotagliflozin, crystalline forms of sotagliflozin, pharmaceutical compositions comprising sotagliflozin, and methods of its manufacture and use; and
Issued patents and pending patent applications in Europe, the United States, and other countries throughout the world, including Australia, Brazil, Canada, China, Europe, India, Israel, Japan, Mexico, New Zealand, South Africa, and South Korea, that claim LX9211, pharmaceutical compositions comprising LX9211, and methods of its use.
The company has filed patent applications and hold issued patents covering each of the company’s drug candidates. None of the company’s United States patents that claim one of the company’s drug candidates has a normal expiration date earlier than 2028.
The Lexicon name and logo are registered trademarks of the company.
Research and Development Expenses
In 2022, the company incurred expenses of $52.8 million in company-sponsored, as well as collaborative research and development activities, including $4.3 million of stock-based compensation expense in 2022.
History
Lexicon Pharmaceuticals, Inc., a Delaware corporation, was founded in 1995. The company was incorporated in 1995.

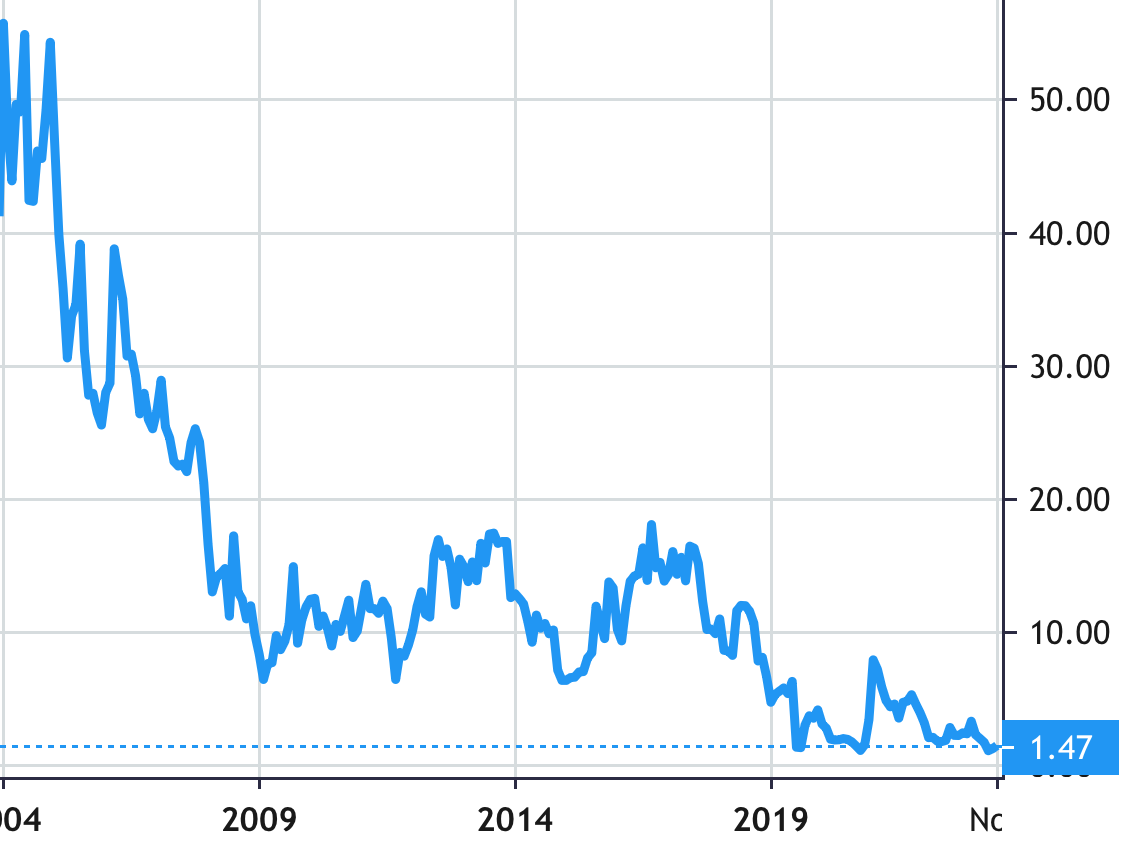
 Stock Value
Stock Value

