About Pulmonx
Pulmonx Corporation, a commercial-stage medical technology company, provides a minimally invasive treatment for patients with severe emphysema, a form of chronic obstructive pulmonary disease (COPD).
The company's solution, which consist of the Zephyr Endobronchial Valve (Zephyr Valve), the Chartis Pulmonary Assessment System (Chartis System) and the StratX Lung Analysis Platform (StratX Platform), is designed to treat severe emphysema patients who, despite medical management, are still profoundly symptomatic and either do not want or are ineligible for surgical approaches.
In 2018, the company received pre-market approval (PMA) by the U.S. Food and Drug Administration (FDA). The Zephyr Valve is commercially available in more than 25 countries, with over 100,000 valves used to treat more than 25,000 patients. The company has established reimbursement in major markets in North America, Europe and the Asia Pacific and the Zephyr Valve has been included in treatment guidelines for COPD worldwide.
The company markets and sells its products in the United States through a direct sales organization. The company's sales territory managers are focused on promoting awareness and increasing adoption of its solution primarily among the pulmonologists performing interventional pulmonary procedures across the United States. The company is expanding its commercial operations in the United States while continuing to foster its international growth. In international markets, it employs both direct and distributor-based sales models, with over 96% of its revenue generated in markets where it sells directly for the year ended December 31, 2023. The company generates most of its revenue from the sales of Zephyr Valves and delivery catheters. The company also generates a smaller amount of its revenue from its Chartis System, which consists of sales of the balloon catheters, usage fees and sales of the Chartis console. The StratX Platform, while used to identify patients eligible for treatment with Zephyr Valves, does not independently generate any revenue for it.
Solution
The company's solution, which cosnists of the Zephyr Valve, Chartis System and StratX Platform, is designed to treat severe emphysema patients who, despite medical management, are still profoundly symptomatic and either do not want or are ineligible for surgical approaches.

Zephyr Valves
Each of the Zephyr Valves consists of a one-way silicone duckbill valve suspended inside a self-expanding frame made of shape-memory metal, called Nitinol. The Zephyr Valve is designed to be easily and accurately sized and offers controlled and accurate deployment at the target location. The Zephyr Valve is also designed to resist fractures or breakage, adapt to changes in airway size and stay in place following deployment.
Zephyr Valves offer a controlled, stepwise deployment for easy and accurate placement in the target airway. Once deployed, the valve is held in place by the radial expansion force of the housing. Typically, multiple valves are used to obstruct all airways leading to the target lobe; in clinical studies, an average of four valves per patient were used.
The Zephyr Valve is designed to be a permanent implant, but unlike surgery, the procedure can be reversed if necessary.
StratX Platform
The StratX Platform is a cloud-based quantitative CT analysis service that provides physicians with an easy-to-read report that the company designed for its solution that includes information on emphysema destruction, fissure completeness and lobar volume to help identify target lobes for treatment with Zephyr Valves. After the physician captures a CT scan of the patient's chest according to the StratX parameters, the CT scan is de-identified of patient information, and the hospital staff uploads the CT scan to a third-party cloud service provider where it is analyzed using validated algorithms within the StratX Platform. The StratX Platform is designed to enable physicians to screen treatment candidates non-invasively, prioritize between multiple potential treatment targets, if applicable, enhance case planning, and educate themselves and their patients using the simple-to-read StratX Lung Report.
In order to make the StratX Platform available to physicians, the company contracts with a third-party cloud service provider. This third-party cloud service enables physicians to upload CT scan data while removing protected health information (PHI) of patients from that data, in case the physicians have, inadvertently, not removed the PHI themselves. The company also contracts with additional third-party service providers to analyze the CT scan data using their proprietary software and provide quantitative results via the StratX Lung Report. The StratX Lung Report is then made available to physicians in the third-party cloud service. The software of each of these third-party service providers has received either 510(k) approval or successfully underwent a conformity assessment procedure with a Notified Body and was subsequently CE Marked in accordance with applicable legislation governing medical devices. The company provides exclusive access to physicians to their StratX accounts and cases and monitor this CT scan upload and analysis process to ensure quality control.
Chartis Pulmonary Assessment System
The Chartis System is a proprietary balloon catheter and console system with flow and pressure sensors designed to assess the presence of collateral ventilation and has been validated in multiple randomized controlled clinical trials to predict likely responders to Zephyr Valve treatment. The Chartis System consists of a single-patient-use catheter with a central lumen and a balloon at its tip and a console to allow for the assessment of airflow in the targeted lobe.
The Chartis System offers a physiologic technique for measuring collateral ventilation and complements non-invasive estimates of fissure completeness. Other methods, such as using fissure analysis as a proxy measurement of collateral ventilation allows detection of an incomplete boundary between the lobes but does not measure how much air is flowing across this gap. Without assessment by the Chartis System, physicians may treat a lobe that has collateral ventilation, which will likely not respond to valve treatment, or unnecessarily rule out a patient who could have potentially benefitted from valve treatment.
Commercial Strategy
The company has established a stepwise approach to market development which centers on active engagement across three key stakeholders in addressing severe emphysema: hospitals, physicians and patients.
The company sells Zephyr Valves primarily through a direct sales force that engages with pulmonologists in the United States, Europe and the Asia Pacific. Zephyr Valves are typically implanted by an interventional pulmonologist at a hospital, and patients are often evaluated in a multi-disciplinary team approach that includes other lung physicians, radiologists, respiratory therapy specialists and/or surgeons. The company's sales personnel work closely with these stakeholders to ensure quality outcomes. The company offers an in-depth training program developed in conjunction with leading global thought leaders and the largest pulmonary society in the United States. The company's sales personnel work with hospitals to leverage their existing resources to efficiently establish and market Zephyr Valves as a service line. The company's sales territory managers also call on community physicians, nurses, respiratory therapists and pulmonary rehabilitation centers to raise awareness of Zephyr Valves as a treatment option.
The company's strategy is to identify territories with high unmet need, identify leading hospitals and work with champions of its solution to establish quality Zephyr Valve programs. The company facilitates sharing of best practices among hospitals on how to efficiently educate stakeholders, screen patients, and manage patient care.
The company intends to continue to promote awareness of its solution through training and educating physicians, pulmonary rehabilitation centers, key opinion leaders, various medical societies, and prospective patients on the proven clinical benefits of Zephyr Valves. The company continues to develop its relationships with credible third parties, such as its partnership with the American College of Chest Physicians and Medscape, on continuing medical education-accredited training and with the American Lung Association and the COPD Foundation on patient and physician education. In addition, the company intends to continue to publish additional clinical data in various industry and scientific journals, online and through presentations at various industry conferences.
The company conducts its international business through direct sales in markets with established reimbursement and substantial market potential, and through a distributor-based sales model in smaller markets or markets where it is still developing reimbursement.
Third-Party Reimbursement
The company's patient access team is responsible for all aspects of its reimbursement processes and initiatives. In the United States, the company's solution is reimbursed based on established Category I CPT and ICD-10 PCS codes and associated APC and MS-DRG payment groupings.
Coding
In the United States, the company sells its products to hospitals. These customers in turn bill various third-party payors, such as commercial payors and government agencies, for the cost required to treat each patient.
Third-party payors require physicians and hospitals to identify the items and services for which they are seeking reimbursement by using standard codes for both physician and facility payments.
Research, Development and Clinical Programs
The company's research and development team continues to design, develop and test new innovations to improve patient outcomes and expand its addressable market. The company also works with external vendors in the design and testing of new technologies.
The company's pipeline of products that it is considering includes innovations in image analysis to support advanced patient selection and optimize patient outcomes, catheter technologies to improve Chartis assessment, valve deliverability and reduce procedure time and the use of AeriSeal for addressing the needs of severe emphysema patients who are not eligible for Zephyr Valves due to collateral ventilation.
AeriSeal is a polymerizing sealant that can be delivered via a bronchoscope to a targeted region of the lung to reduce volume in the treated area. AeriSeal would enable the treatment of patients with collateral ventilation, which would complement the screening of patients for Zephyr Valves. The company has successfully undertaken the conformity assessment procedure in the EU with a Notified Body and CE marked AeriSeal on the basis of the MDD (as defined below), which it continues to place on the market in accordance with the transitional provisions of the MDR (as defined below), and has Therapeutic Goods Administration approval in Australia for the medical device and have completed initial feasibility research. The company has received a staged IDE approval to commence a clinical trial with AeriSeal.
Intellectual Property
As of December 31, 2023, the company had 35 patent families in force worldwide. As of December 31, 2023, the company had rights to 65 issued United States patents, 15 pending United States patent applications, 112 issued foreign patents and 15 pending foreign patent applications. The company's most material foreign patents issued and patent applications pending are in the European Union (EU), France, Germany, Japan and the United Kingdom. The company's patents cover aspects of its current Zephyr Valve, loading system, airway sizing, EDC, Chartis System, AeriSeal, StratX, and future product concepts. The term of individual patents depends on the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a nonprovisional patent application in the applicable country. The company's patents expire between 2024 and 2041. Once a patent expires, the protection ends, and an invention enters the public domain; that is, anyone can commercially exploit the invention without infringing the patent.
The company also relies upon trademarks to build and maintain the integrity of its brand. As of December 31, 2023, the company had nine registered trademarks, some of which apply to multiple countries, and several pending trademark applications in various countries.
Cross-Licensing Agreement with Spiration/Olympus
In 2005, Emphasys Medical (Emphasys), a company the company later acquired, entered into a cross-license agreement (Spiration Cross-License) with Spiration, Inc. (Spiration) (later acquired by Olympus Medical Systems Corp.). Since both companies were developing products in the same field, they entered into this agreement to minimize the risk of intellectual property disputes in the future and their associated cost.
Research and Development
The company's research and development expenses included $18.1 million during the year ended December 31, 2023.
Government Regulation
The company's products and operations are subject to extensive and ongoing regulation by the FDA under the Federal Food, Drug, and Cosmetic Act of 1938 and its implementing regulations (FDCA), as well as other federal and state regulatory bodies in the United States. The laws and regulations govern, among other things, product design and development, pre-clinical and clinical testing, manufacturing, packaging, labeling, storage, record keeping and reporting, clearance or approval, marketing, distribution, promotion, import and export and post-marketing surveillance.
Unless an exemption applies, each new or significantly modified medical device the company seeks to commercially distribute in the United States will require either a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, or approval from the FDA of a PMA application. Both the 510(k) clearance and PMA processes can be resource intensive, expensive and lengthy, and require payment of significant user fees, unless an exemption is available.
The company's portfolio of products is regulated in the European Union as a medical device per the European Union Medical Devices Directive (Council Directive 93/42/EEC) (MDD) and the Medical Device Regulation (Regulation (EU) 2017/745) (MDR).
History
The company was incorporated in the state of California in 1995 and was reincorporated in the state of Delaware in December 2013. It was formerly known as Pulmonx and changed its name to Pulmonx Corporation in 2013.
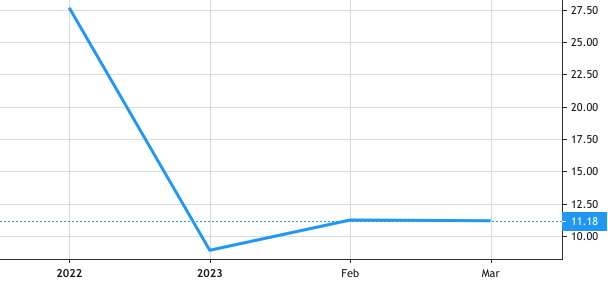
 Stock Value
Stock Value

