About Intra-Cellular Therapies
Intra-Cellular Therapies, Inc. operates as a biopharmaceutical company.
The company focuses on the discovery, clinical development and commercialization of innovative, small molecule drugs that address underserved medical needs primarily in neuropsychiatric and neurological disorders by targeting intracellular signaling mechanisms within the central nervous system, or CNS.
In 2019, CAPLYTA (lumateperone) was approved by the U.S. Food and Drug Administration, or FDA, for the treatment of schizophrenia in adults (42 mg/day) and the company initiated the commercial launch of CAPLYTA in 2020. In 2021, CAPLYTA was approved by the FDA for the treatment of bipolar depression in adults (42 mg/day). The company initiated the commercial launch of CAPLYTA for the treatment of bipolar depression in December 2021. Additionally, in April 2022, the FDA approved two additional dosage strengths of CAPLYTA, 10.5 mg and 21 mg capsules, to provide dosage recommendations for patients concomitantly taking strong or moderate CYP3A4 inhibitors, and 21 mg for patients with moderate or severe hepatic impairment (Child-Pugh class B or C). The company initiated the commercial launch of these special population doses in August 2022. CAPLYTA refers to lumateperone approved by the FDA for the treatment of schizophrenia in adults and for the treatment of bipolar depression in adults, and lumateperone refers to, where applicable, CAPLYTA as well as lumateperone for the treatment of indications beyond schizophrenia and bipolar depression.
Product
In December 2019, CAPLYTA was approved by the FDA for the treatment of schizophrenia in adults (42 mg/day) and the company initiated the commercial launch of CAPLYTA in March 2020. In support of the company’s commercialization efforts, it employs a national sales force. In December 2021, CAPLYTA was approved by the FDA for the treatment of bipolar depression in adults (42 mg/day). CAPLYTA is the only FDA-approved treatment for depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults as monotherapy and as adjunctive therapy with lithium or valproate. The company initiated the commercial launch of CAPLYTA for the treatment of bipolar depression in December 2021. In addition, the FDA approved two additional dosage strengths, 10.5 mg and 21 mg, for special populations of patients, in April 2022. The company initiated the commercial launch for these special population doses in August 2022.
The efficacy of CAPLYTA 42 mg in schizophrenia was demonstrated in two placebo-controlled trials, showing a statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale, or PANSS, total score. The most common adverse reactions (>5% and twice the rate of placebo) for the recommended dose of CAPLYTA versus placebo were somnolence/sedation (24% vs. 10%) and dry mouth (6% vs. 2%). In pooled data from short term studies, mean changes from baseline in weight gain, fasting glucose, triglycerides and total cholesterol were similar between CAPLYTA and placebo. The incidence of extrapyramidal symptoms was 6.7% for CAPLYTA and 6.3% for placebo.

The efficacy of CAPLYTA 42 mg in bipolar depression was demonstrated in two positive Phase 3 placebo-controlled bipolar depression studies, which evaluated the effects of CAPLYTA on depression in adult patients with bipolar I or bipolar II disorder both as monotherapy (Study 404) and as adjunctive therapy with lithium or valproate (Study 402). In these studies, the efficacy of CAPLYTA 42 mg was established by demonstrating statistically significant improvements over placebo for the change from baseline in the Montgomery-Asberg Depression Rating scale, or MADRS, total score at Week 6. CAPLYTA 42 mg also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder in each study.
Development Programs
The company’s pipeline includes several product candidates in clinical development and additional product candidates in non-clinical testing. The company’s product candidates offer innovative therapeutic approaches and may provide advantages relative to current therapies.
Lumateperone Development Program
The efficacy of lumateperone could be mediated through a combination of antagonist activity at central serotonin 5-HT2A receptors and postsynaptic antagonist activity at central dopamine D2 receptors. In terms of pharmacodynamics, lumateperone has high binding affinity for serotonin 5-HT2A receptors and moderate binding affinity for dopamine D2 receptors, serotonin transporters, dopamine D1 receptors, dopamine D4 receptors and adrenergic alpha 1A and alpha 1B receptors. It lacks biologically relevant interactions with other receptors including muscarinic and histaminergic receptors. As a result, lumateperone may represent a potential treatment across multiple therapeutic indications.
Lumateperone for the treatment of major depressive disorder and other mood disorders
As a potent 5-HT2A receptor antagonist and serotonin reuptake inhibitor, lumateperone could improve symptoms of depression. Dopamine modulation by lumateperone may also reduce irritability and aggression that can accompany many mood disorders. Lumateperone, as a standalone agent, indirectly enhances glutamatergic neurotransmission through both AMPA and NMDA channels in the prefrontal cortex via lumateperone’s dopamine D1 receptor activation. By enhancing AMPA neurotransmission, lumateperone also activates key proteins in the mTOR pathway which has shown antidepressant effects. As such, lumateperone may represent a potential treatment for mood disorders including major depressive disorder, or MDD.
Lumateperone is in Phase 3 clinical development as a novel treatment for MDD. Clinical conduct in Study 501, Study 502 and Study 505, global Phase 3 clinical trials evaluating lumateperone 42 mg as an adjunctive therapy to antidepressants for the treatment of MDD, is ongoing. Study 505 is intended to serve as a potential additional registration trial in support of a supplemental New Drug Application, or sNDA, for approval of lumateperone as an adjunctive therapy to antidepressants for the treatment of MDD, if needed. This is a common strategy employed in mood disorder development programs. The company expects to announce topline results from Study 501 in April of 2024 and Study 502 late in the second quarter of 2024 and, subject to the results of these studies, the company expects to file an sNDA with the FDA for approval of lumateperone as an adjunctive therapy to antidepressants for the treatment of MDD in the second half of 2024.
In 2020, as part of its lumateperone bipolar depression clinical program, the company initiated its third monotherapy Phase 3 study, Study 403, evaluating lumateperone as monotherapy in the treatment of major depressive episodes associated with bipolar I or bipolar II disorder. Following the positive results in its adjunctive study that was part of its bipolar depression clinical program, Study 402, it amended Study 403 to evaluate major depressive episodes with mixed features in bipolar disorder in patients with bipolar I or bipolar II disorder and mixed features in patients with MDD. In March 2023, the company announced positive topline results from Study 403 as lumateperone 42 mg given once daily met the primary endpoint in the study by demonstrating a statistically significant and clinically meaningful reduction in the Montgomery-Asberg Depression Rating Scale (MADRS) total score compared to placebo at Week 6 in the combined patient population of MDD with mixed features and bipolar depression with mixed features (5.7 point reduction vs. placebo; p<0.0001; Cohen’s d effect size (ES) of 0.64).
The company also has an ongoing study, Study 304, evaluating lumateperone for the prevention of relapse in patients with schizophrenia. The study is being conducted in five phases consisting of a screening phase; a 6-week, open-label run-in phase during which all patients will receive 42 mg of lumateperone per day; a 12-week, open-label stabilization phase during which all patients will receive 42 mg of lumateperone per day; a double-blind treatment phase, 26 weeks in duration, during which patients receive either 42 mg of lumateperone per day or placebo (1:1 ratio); and a 2-week safety follow-up phase. This study is being conducted in accordance with its post approval marketing commitment to the FDA in connection with the approval of CAPLYTA for the treatment of schizophrenia as is typical for antipsychotics.
Other Indications for Lumateperone
Within the lumateperone portfolio, the company is conducting, or are in the process of initiating, studies with pediatric patients in schizophrenia, bipolar disorder and irritability associated with autism spectrum disorder. In addition, the company is developing a long-acting injectable, or LAI, formulation to provide more treatment options to patients suffering from mental illness. The company has conducted a Phase 1 single ascending dose study with an LAI formulation. This study evaluated the pharmacokinetics, safety and tolerability of a lumateperone LAI in patients with stable symptoms of schizophrenia and was generally safe and well-tolerated. The company is evaluating several additional formulations of a lumateperone LAI with treatment durations of one month and longer. The company has completed all non-clinical studies to support the initiation of a Phase 1 study with four additional formulations of its LAI. The company expects to commence clinical conduct in this study in the first half of 2024.
The company holds exclusive, worldwide commercialization rights to lumateperone and a family of compounds from Bristol-Myers Squibb Company pursuant to an exclusive license.
Other Product Candidates
The company is developing ITI-1284-ODT-SL for the treatment of generalized anxiety disorder, the treatment of agitation in patients with dementia, and the treatment of dementia-related psychosis. ITI-1284-ODT-SL is a deuterated form of lumateperone, a new molecular entity formulated as an oral disintegrating tablet for sublingual administration. ITI-1284-ODT-SL is formulated as an oral solid dosage form that dissolves almost instantly when placed under the tongue, allowing for ease of use in the elderly and may be particularly beneficial for patients who have difficulty swallowing conventional tablets. Phase 1 single and multiple ascending dose studies in healthy volunteers and healthy elderly volunteers (> than 65 years of age) evaluated the safety, tolerability and pharmacokinetics of ITI-1284-ODT-SL. In these studies, there were no reported serious adverse events in either age group. In the elderly cohort, reported adverse events were infrequent with the most common adverse event being transient dry mouth (mild). Based on these results, the company has initiated Phase 2 programs evaluating ITI-1284-ODT-SL for the treatment of generalized anxiety disorder, psychosis in Alzheimer's disease and agitation in patients with Alzheimer’s disease. As a result, the non-clinical data from lumateperone may not be broadly applied to ITI-1284-ODT-SL, and the company conducted additional toxicology studies. The company is continuing with Phase 1 studies with ITI-1284-ODT-SL, including drug-drug interaction studies.
The company has another major program that has yielded a portfolio of compounds that selectively inhibit the enzyme phosphodiesterase type 1, or PDE1. PDE1 enzymes are highly active in multiple disease states, and its PDE1 inhibitors are designed to reestablish normal function in these disease states. Abnormal PDE1 activity is associated with cellular proliferation and activation of inflammatory cells. The company’s PDE1 inhibitors ameliorate both of these effects in animal models. The company intends to pursue the development of its phosphodiesterase, or PDE, program, for the treatment of aberrant immune system activation in several CNS and non-CNS conditions with a focus on diseases where excessive PDE1 activity has been demonstrated and increased inflammation is an important contributor to disease pathogenesis. The company’s potential disease targets include immune system regulation, neurodegenerative diseases, cancers and other non-CNS disorders. Lenrispodun (ITI-214) is its lead compound in this program. Following the favorable safety and tolerability results in its Phase 1 program, the company initiated its development program for lenrispodun for Parkinson’s disease and conducted a Phase 1/2 clinical trial of lenrispodun in patients with Parkinson’s disease to evaluate safety and tolerability in this patient population, as well as motor and non-motor exploratory endpoints. In this study, lenrispodun was generally well-tolerated with a favorable safety profile and clinical signs consistent with improvements in motor symptoms and dyskinesias. The company’s Phase 2 clinical trial of lenrispodun evaluating improvements in motor symptoms, changes in cognition, and inflammatory biomarkers in patients with Parkinson’s disease is ongoing. The company expects to complete patient enrollment in this study in late 2024 with topline results anticipated in the first half of 2025. The company also has an active Investigational New Drug application to evaluate its newest candidate within the PDE 1 inhibitor program, ITI-1020, as a novel cancer immunotherapy. The company’s Phase 1 program with ITI-1020 in healthy volunteers is ongoing.
The company has a development program with its ITI-333 compound as a potential treatment for substance use disorders, pain and psychiatric comorbidities including depression and anxiety. There is a pressing need to develop new drugs to treat opioid addiction and safe, effective, non-addictive treatments to manage pain. ITI-333 is a novel compound that uniquely combines activity as an antagonist at serotonin 5-HT2A receptors and a partial agonist at µ-opioid receptors. These combined actions support the potential utility of ITI-333 in the treatment of opioid use disorder and associated comorbidities (e.g., depression, anxiety, sleep disorders) without opioid-like safety and tolerability concerns. The company has conducted a Phase 1 single ascending dose study evaluating the safety, tolerability and pharmacokinetics of ITI-333 in healthy volunteers. In this study, ITI-333 achieved plasma exposures at or above those required for efficacy and was generally safe and well-tolerated. The company has commenced a neuroimaging study to investigate brain occupancy for receptors that play a role in substance use disorder and also have applicability for pain. The results of this study will support the dose selection for future studies. The company also has an ongoing multiple ascending dose study with ITI-333 in healthy volunteers. The company has received a grant from the National Institute on Drug Abuse under the Helping to End Addiction Long-term Initiative, or NIH HEAL Initiative.
The company also has its ITI-1500 program focused on the development of novel non-hallucinogenic psychedelics. Compounds in this series interact with serotonergic (5-HT2a) receptors in a unique way, potentially allowing the development of this new drug class in mood, anxiety and other neuropsychiatric disorders without the known limitations of psychedelics including the hallucinogenic potential and risk for cardiac valvular pathologies. The company’s lead compound in this program, ITI-1549, is being evaluated in IND enabling studies.
Drug Discovery Platform and Capabilities
Based on the pioneering efforts of its late co-founder and Nobel laureate, Dr. Paul Greengard, the company has developed a detailed understanding of intracellular signaling pathways and intracellular targets. The company has used that knowledge to develop several state of the art technology platforms, including one called CNSProfile. This technology monitors the phosphoprotein changes elicited by major psychotropic drug classes and subclasses, and generates a unique molecular signature for drug compounds. By monitoring how the levels of these phosphoproteins change in vivo, the company identifies intracellular signaling pathways through which several major drug classes operate.
Strategy
Using its key understanding of intracellular signaling, the company seeks to accomplish its goal, using its in-house expert drug discovery and clinical development teams, in two ways:
The company seeks to have the capability to develop first-in-class medications with novel mechanisms that have the potential to treat CNS diseases and other diseases for which there are no previously marketed drugs; and
The company seeks to develop drugs that either can differentiate themselves in competitive markets by addressing aspects of CNS diseases and other diseases which are not adequately treated by marketed drugs or can be effective with fewer side effects.
The key elements of the company’s strategy are to continue to commercialize CAPLYTA, which has been approved by the FDA for the treatment of schizophrenia and bipolar depression in adults, in the United States; complete the development of lumateperone for additional neuropsychiatric indications, such as MDD; expand the commercial potential of lumateperone by investigating its usefulness in additional neurological areas; continue to advance its other product candidates in clinical development, such as PDE1 inhibitors, including lenrispodun for the treatment of CNS and other disorders; ITI-1284, for the treatment of generalized anxiety disorder, psychosis in Alzheimer's disease and agitation in patients with Alzheimer's disease; and ITI-333, for substance use disorders, pain and psychiatric comorbidities including depression and anxiety; and advance the earlier stage product candidates in its pipeline, such as ITI-1500, for mood and other neuropsychiatric disorders.
Intellectual Property
Patent Portfolio
As of February 1, 2024, the company owned or controlled approximately 134 patent families filed in the United States and other major markets worldwide, including approximately 136 issued or allowed U.S. patents, 70 pending U.S. patent applications, 563 issued or allowed foreign patents and 335 pending foreign patent applications, directed to novel compounds, formulations, methods of treatment, synthetic methods, and platform technologies.
Lumateperone tosylate is FDA-approved as CAPLYTA for the treatment of schizophrenia and for the treatment of bipolar depression. The company has extensively characterized this compound and related compounds and filed additional patent applications on salt forms, polymorphs, pharmaceutical formulations, new indications, improved methods of manufacture, metabolites, derivatives, and structurally related novel compounds. As of February 1, 2024, the company’s lumateperone program consisted of approximately 39 patent families that it owns or controls, filed in the United States and other major markets, including 56 issued or allowed U.S. patents, 34 pending U.S. patent applications, 240 issued or allowed foreign patents and 143 pending foreign patent applications. 18 patents are currently Orange Book listed in the United States. During 2023, the RE48,839 patent was selected for Patent Term Extension, extending its term by 215 days, through August 2033. Other pending U.S. and foreign patent applications may protect additional indications and formulations through 2043. In addition to patent protection, lumateperone has five years of new chemical entity data exclusivity with the FDA, until December 2024.
The company’s ITI-1284 program relates to novel lumateperone derivatives for the treatment of behavioral disturbances associated with dementia, and other central nervous system disorders. Eight families of patent applications have been filed which provide coverage for ITI-1284, which have already resulted in 10 U.S. patents and 70 foreign patents. The ITI-1284 molecule has composition of matter protection to 2037, with possible extensions and additional Orange Book-listable protection to 2042.
The company’s program on PDE1 inhibitors for cognition, dopamine-mediated and other disorders, as well as several others, includes patent protection across 57 families, including 31 families for the lead molecule, lenrispodun, as well as a wide range of filings on other proprietary compounds and indications. The lenrispodun lead molecule has composition of matter protection to 2029, with possible extensions and additional Orange Book-listable protection to 2034. Additionally, the company expects to have data exclusivity in the European Union (EU) for up to 11 years from commercial launch. The company is also evaluating potential follow-on compounds for lenrispodun which would have patent protection beyond 2030.
The company’s ITI-333 program relates to novel compounds for the non-addictive treatment of pain and for the treatment of opiate use disorder. 18 families of patent applications have been filed, including six families which have already resulted in eight U.S. patents and 69 foreign patents. These patent families will protect the lead compound, as well as many other analogs under development, beyond 2037 (exclusive of any patent term extensions and regulatory exclusivities).
The company’s early-stage ITI-1500 program relates to novel compounds as non-hallucinogenic psychedelics for the treatment of a variety neuropsychiatric disorders, including depression and anxiety. Composition of matter patent protection will extend to at least 2043, with possible extensions and additional Orange Book-listable protection to 2048.
The company has also filed patent applications on novel proprietary targets and lead compounds for AD, which would provide compound protection beyond 2028 or beyond 2034, depending on which compound is ultimately selected for development.
License Agreement
The Bristol-Myers Squibb License Agreement
In 2005, the company entered into a worldwide, exclusive License Agreement with Bristol-Myers Squibb Company, or BMS, pursuant to which it holds a license to certain patents and know-how of BMS relating to lumateperone and other specified compounds. The agreement was amended on November 3, 2010. The licensed rights are exclusive, except BMS retains rights in specified compounds in the fields of obesity, diabetes, metabolic syndrome and cardiovascular disease. However, BMS has no right to use, develop or commercialize lumateperone and other specified compounds in any field of use. The company has the right to grant sublicenses of the rights conveyed by BMS.
Manufacturing
The Siegfried Supply Agreement
In 2017, the company entered into a supply agreement with Siegfried Evionnaz SA, an affiliate of Siegfried AG, or Siegfried. Following the automatic expiration of the agreement on January 4, 2023, it entered into a new supply agreement with Siegfried effective January 5, 2023, or the Siegfried Agreement. Under the Siegfried Agreement, Siegfried has agreed to manufacture and supply the API for lumateperone in commercial quantities. The company agreed to provide Siegfried with a rolling forecast of its anticipated requirements for supply of the API. The initial term of the Siegfried Agreement is three years until January 5, 2026. The Siegfried Agreement will automatically renew on an evergreen basis for a consecutive one-year period, unless either party notifies the other party of its election to not renew the agreement at least 12 months prior to the end of the initial term or any renewal period then in effect. As of December 31, 2023, the company had committed to purchasing production campaigns of API and intermediate product from Siegfried that are expected to be delivered in 2024.
The Lonza Manufacturing Services Agreement
In 2017, the company entered into a manufacturing services agreement, as amended on December 19, 2022, or the Lonza Agreement, with Lonza Ltd., or Lonza. Under the Lonza Agreement, Lonza has agreed to manufacture and supply the API for lumateperone, with purchase prices specified based on the volume produced. On December 19, 2022, the Lonza Agreement was amended to, among other items, extend the current term of the agreement until December 31, 2028 and provide for certain minimum annual purchase commitments by the company for the years 2025 through 2028 for delivery in 2026 through 2029. Either party may terminate the agreement prior to its expiration upon a prior written notice, an uncured material breach by the other party, the insolvency, liquidation, dissolution or bankruptcy of the other party, or a continuing force majeure event affecting the other party, and may be extended by mutual consent. As of December 31, 2023, the company had committed to purchasing production campaigns of API from Lonza that are expected to be delivered in 2024 and 2025.
Commercial Operations
The company initiated the commercial launch of CAPLYTA for the treatment of schizophrenia in adults in the United States in 2020. In 2021, the company launched CAPLYTA for the treatment of bipolar depression in adults. In support of its commercialization efforts, the company employs a national sales force. In the future, the company may choose to commercialize CAPLYTA or any other products, in markets outside of the United States, if approved for sale in such markets, by establishing one or more strategic alliances.
Customers
The company is approved to sell CAPLYTA for the treatment of schizophrenia in adults and for the treatment of bipolar depression in adults in the United States. CAPLYTA is priced in line with other marketed branded antipsychotics indicated for the treatment of schizophrenia and bipolar depression. The company distributes CAPLYTA principally through three third-party wholesale drug distributors whose concentration are each between 29% and 36% of total sales.
Government Regulation
The company’s commercial marketing of CAPLYTA and related business activities could become subject to scrutiny and enforcement under one or more federal or state health care fraud and abuse laws and regulations. These fraud and abuse laws, and other applicable health care laws include:
The federal Anti-Kickback Law, which prohibits, among other things, knowingly or willingly offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any goods or services for which payment may be made, in whole or in part, by federal health care programs such as Medicare and Medicaid;
The federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government, and which provides for civil whistleblower or qui tam actions against individuals or entities alleged to have submitted a false claim;
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal liability for knowingly and willfully executing a scheme to defraud any healthcare benefit program, knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense, or knowingly and willfully making false statements relating to healthcare matters;
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
The federal Physician Payment Sunshine Act, which requires manufacturers of FDA-approved drugs (among others) covered by Medicare or Medicaid to report to CMS, on an annual basis, information related to payments and other transfers of value to physicians, teaching hospitals, and certain advanced non-physician health care practitioners and physician ownership and investment interests, with such data then being made publicly available on a website maintained by CMS called Open Payments; and
Analogous state and foreign laws and regulations, including state anti-kickback and false claims laws, which may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the payer, as well as other state laws that require pharmaceutical companies to report expenses related to the marketing and promotion of pharmaceutical products, prohibit certain gifts or payments to health care providers in the state, and/or require pharmaceutical companies to implement compliance programs or marketing codes of conduct.
Research and Development
The company’s research and development expenses were $180.1 million for the year ended December 31, 2023.
Competition
CAPLYTA for the treatment of schizophrenia and for the treatment of bipolar depression competes with, among other branded products, Fanapt, marketed by Vanda Pharmaceuticals; Lybalvi, marketed by Alkermes; Rexulti, marketed by Otsuka Pharmaceutical; and Vraylar, marketed by AbbVie.
History
Intra-Cellular Therapies, Inc. was founded in 2002. The company was incorporated in the state of Delaware in 2012.
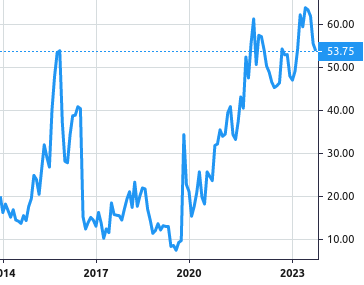
 Stock Value
Stock Value

