About IGM Biosciences
IGM Biosciences, Inc., a clinical-stage biotechnology company, engages in the development of immunoglobulin M (IgM) antibodies for the treatment of cancer, autoimmune and inflammatory diseases, and infectious diseases.
The company has created a proprietary IgM antibody technology platform that is particularly well suited for developing receptor cross-linking agonists, T cell engagers, targeted cytokines, and target neutralizers.
The company created its IgM platform to expand upon the inherent qualities of IgM antibodies and to allow for the rapid development of engineered therapeutic antibodies. The company’s IgM platform creates significant competitive advantages and can serve as the foundation for the development of a broad range of IgM based therapeutic drugs.
Antibodies
The company created its IgM platform to expand upon the inherent properties of IgM antibodies and to allow for the rapid development of engineered therapeutic antibodies. Significantly, the company’s IgM platform allows it to create IgM antibodies with higher affinity and avidity than naturally occurring IgM antibodies.
The company’s platform also provides it the ability to develop engineered IgM antibodies against various targets which enables the creation of a broad and differentiated product pipeline. The company’s initial efforts are focused on four broad applications of IgM antibodies:

Receptor Cross-linking Agonists
The company is also using its IgM platform to develop IgM antibodies that bind to members of the Tumor Necrosis Factor receptor Superfamily (TNFrSF). Members of the TNFrSF must be bound in clusters of at least three in order to send a strong biological signal to the cell. This family includes targets that will cause the death of cancer cells, such as DR5, and targets that will cause the proliferation of T cells.
There have been multiple attempts to create immunoglobulin G (IgG) based therapeutic antibodies directed at DR5 and other members of the TNFrSF. However, since IgG antibodies naturally bind only two cell surface proteins, their bivalent nature inherently limits their signaling efficacy with this class of targets. In contrast, the company is utilizing the 10 binding domains of IgM antibodies to more efficiently cross-link these molecules on the cell surface. In multiple in vitro cell studies, the company has observed that IgM antibodies have much greater potency than IgG antibodies with the same binding domains against this class of targets.
T cell Engagers
The company has been able to utilize the natural features of IgM antibodies to create unique and patent protected bispecific T cell engagers, which may have the potential to kill cancer cells through T cell directed cellular cytotoxicity (TDCC) and CDC while maintaining a favorable tolerability profile. Bispecific T cell engagers, including CD20 x CD3, CD123 x CD3, CD38 x CD3 and solid tumor target x CD3, are designed to simultaneously target a desired tumor associated antigen on a cancer cell and CD3 (a protein that is expressed on the surface of T cells) and redirect the T cells to kill the cancer cells, a form of TDCC. In contrast to other bispecific antibody formats that bind to one or two target molecules on the surface of the cancer cell and to one CD3 molecule on the surface of the T cell, the company’s IgM bispecific format includes 10 binding domains to the target on the surface of the cancer cell and one binding domain to CD3.
Targeted Cytokines
The company is using its IgM platform to create bifunctional IgM antibodies with high avidity to selected cell surface targets to deliver potent, immune stimulating cytokines. These IgM antibodies are designed to target the delivery of IL-15 to induce immune cell stimulation and proliferation. Targeted delivery of cytokines is designed to reduce systemic toxicities of cytokine therapy while enhancing immune system activity in the tumor microenvironment. Stimulation of the IL-15 pathway may be important in strengthening and maintaining both the endogenous and the synthetic T cell immune responses.
Target Neutralizer
The company is also applying its IgM platform to infectious diseases as the high avidity of IgM antibodies may provide improved virus neutralization and reduce antibody evasion through viral mutation. For COVID-19, the company’s initial development focus in infectious diseases, it has observed in multiple in vitro and in vivo studies that IgM antibodies achieve more potent neutralization of SARS-CoV-2 compared to IgG antibodies with the same binding domains. The company also intends to leverage the multi-valent structure of an IgM platform to develop a novel viral trapping approach that has the potential to offer resilience against viral evolution for SARS family viruses.
Development Programs
IGM-8444: Death Receptor 5 Agonist IgM Antibody
IGM-8444, one of the company’s clinical-stage product candidates, is an IgM antibody targeting Death Receptor 5 (DR5) for the treatment of patients with solid and hematologic malignancies. DR5 is a member of the TNFrSF and is often expressed on the surface of cancer cells. Similar to other members of the TNFrSF, strong signaling to effect a biological response requires that three or more DR5 receptor proteins be cross-linked together on the surface of a cancer cell through the binding of either the natural DR5 ligand (TRAIL) or an antibody or other therapeutic drug that can efficiently cross-link the DR5 receptors. Binding and cross-linking of DR5 receptors send a signal to the cancer cell to induce programmed death of cancer cells, also known as apoptosis.
DR5 is expressed in a broad range of solid tumors (e.g., colon, gastric, pancreatic, lung, breast and prostate tumors), as well as leukemias and lymphomas. Although DR5 is expressed on some normal cells in the body, cancer cells have been shown to be more sensitive to DR5 signaling compared to cells of healthy tissues. Various IgG DR5 antibodies have been tested in early stage clinical trials by other companies, but these IgG antibodies failed to demonstrate adequate efficacy. As IgG DR5 antibodies only bind to two DR5 receptors, these IgG antibodies may not have created sufficient cross-linking of DR5 to send an efficient apoptotic signal to the cancer cells, which may account for the relatively small number of monotherapy responses observed in the clinical trials of these IgG antibodies. In contrast, DR5 IgM antibodies have the capacity for multivalent binding of DR5, which results in cross-linked DR5 receptors on the cell surface. IGM-8444 demonstrated significantly enhanced apoptotic signaling compared to an IgG antibody with the same binding domains, resulting in >1,000 fold increased potency in killing cancer cells from multiple cancer cell types in the company’s studies outside of living organisms (in vitro). In the company’s studies in living organisms (in vivo), specifically cynomolgus monkeys, no untoward toxicity was observed with IGM-8444.
Phase 1a/1b Study of IGM-8444 as a Single Agent and in Combination in Patients with Solid and Hematologic Malignancies: The company is conducting the first-in-human, Phase 1a/1b, multicenter, open-label clinical trial to evaluate the safety, tolerability, and pharmacokinetics of IGM-8444 as a single agent and in combination with other agents in subjects with relapsed and/or refractory solid or hematologic cancers. The Phase 1a portion of the trial consists of IGM-8444 dosed intravenously with IGM-8444 as a single agent, IGM-8444 in combination with FOLFIRI +/- bevacizumab, IGM-8444 in combination with birinapant, and IGM-8444 in combination with venetoclax.
The company is also conducting a randomized combination study in patients with colorectal cancer. This combination study is a global randomized trial in second line patients with colorectal cancer who have previously not received FOLFIRI treatment to assess the additional benefit of IGM-8444 combined with the current standard of care regimen of FOLFIRI and bevacizumab. The study endpoints include progression free survival, overall survival, overall response rate, and safety of IGM-8444 in combination with FOLFIRI and bevacizumab.
Imvotamab: CD20 x CD3 Bispecific IgM Antibody
Imvotamab, another of the company’s clinical-stage product candidates, is a CD20 x CD3 bispecific IgM antibody. CD20 is a protein that is frequently expressed on the surface of B cells, while CD3 is a protein that is expressed on the surface of T cells and is an essential activating molecule for the T cell. Imvotamab has 10 binding domains to CD20 and a single binding domain to CD3. In addition, imvotamab contains a human serum albumin molecule attached to the Joining chain (J chain) to enhance its pharmacokinetic properties. The J chain naturally occurs in IgM antibodies and joins the IgM subunits into pentameric antibodies.
Imvotamab is designed to bind a CD20 expressing cell, such as a cancer cell or a pathogenic B cell in autoimmunity, as well as CD3 on a cytotoxic T cell, bringing both cells into close proximity. This interaction mimics the normal T cell activation pathway leading the T cell to recognize and kill the CD20 expressing cell by releasing cytotoxic biochemicals (perforins and granzymes) that penetrate and perforate the CD20 expressing cell. Imvotamab is planned for evaluation in autoimmune diseases mediated by autoreactive antibodies and has been in development for the treatment of cancers expressing CD20.
Imvotamab in Autoimmune Disease
Preclinical studies show that imvotamab penetrates and depletes CD20 expressing cells deep within various tissues, including bone marrow, spleen, and lymph nodes. Additionally, in vitro studies show that imvotamab is significantly more effective than rituximab in depleting low CD20 expressing cells. The company plans to evaluate imvotamab in multiple autoimmune diseases, beginning with Phase 1 studies in systemic lupus erythematosus (SLE) and rheumatoid arthritis.
Imvotamab in Oncology
Imvotamab is also designed to bind to CD20 expressing cancer cells, as well as CD3 on a cytotoxic T cell, to mimic the normal T cell activation pathway, leading the T cell to recognize and kill the CD20 expressing cancer cell by releasing cytotoxic biochemicals (perforins and granzymes) that penetrate and perforate the cancer cell. The company has been developing imvotamab as a treatment for patients diagnosed with CD20-expressing malignancies.
Phase 1/2 Study of Imvotamab in Patients with Relapsed and/or Refractory (R/R) Non-Hodgkin’s Lymphoma (NHL): The company has been conducting Phase 1 and Phase 2, multicenter, open-label clinical trials to evaluate the safety, tolerability, and pharmacokinetics of imvotamab as a monotherapy single agent in subjects with relapsed and/or refractory NHL. For strategic reasons, the company has decided to conclude these studies and not proceed with further clinical development of imvotamab as a monotherapy for NHL. However, the safety and efficacy profile that imvotamab has shown in these Phase 1 and Phase 2 studies may make it an attractive combination partner for use with other active agents in treating NHL, and the company plans to evaluate potential combination agents and partnerships.
IGM-7354: IL-15 x PD-L1 IgM Antibody
IGM-7354, another of the company’s clinical-stage product candidates, consists of an interleukin-15 (IL-15) cytokine molecule and an IL-15 receptor displayed on the J chain of an IgM antibody with the goal of displaying the IL-15 molecules on the surface of PD-L1 expressing cells. This product candidate is intended for the treatment of patients with solid and hematologic malignancies. In nature, IL-15 stimulates T cells and NK cells to proliferate and maintain their long-term survival. The company’s IgM platform allows it to attach IL-15 and an IL-15 receptor to the J chain of an IgM antibody which is designed to bind to a target on the surface of a cell.
Phase 1 Study of IGM-7354 in Patients with Solid Tumor Cancers: The company is conducting a Phase 1 multicenter, open-label clinical trial to evaluate the safety, tolerability, and pharmacokinetics of IGM-7354 as a monotherapy in patients with relapsed and/or refractory solid tumor cancers. The Phase 1 study consists of a dose escalation stage and dose expansion stage, in which IGM-7354 will be intravenously administered.
IGM-2644: CD38 x CD3 Bispecific IgM Antibody
IGM-2644 is a bispecific T cell engaging IgM antibody that targets CD38 and CD3 proteins simultaneously. CD38 is a protein that is frequently and highly expressed on the surface of multiple myeloma cells (a plasma cell cancer), while CD3 is a protein that is expressed on the surface of T cells and is an essential activating molecule required to induce T cell mediated-cytotoxicity.
IGM-2644 with its 10 binding units for CD38 may bind to CD38 expressing cancer cells stronger (higher avidity) compared to an IgG bispecific antibody with only one binding unit for CD38. This may enhance efficacy in patients that develop resistance to anti-CD38 IgG antibodies due to downregulation of CD38. In addition to T cell mediated-cytotoxicity, IGM-2644 can induce complement mediated cytotoxicity which may induce cancer cell death in the absence of T cells. IGM-2644 is also being considered in autoimmune diseases, given that CD38 is expressed on plasma cells which are the source of autoreactive antibodies in several autoimmune diseases. The company expects to initiate the first clinical trial of IGM-2644 in multiple myeloma in 2023.
Additional Pipeline Candidate
The company’s pipeline also includes:
IGM-2537, a bispecific T cell engaging IgM antibody targeting CD123 and CD3 proteins simultaneously and is being developed for the treatment of patients with CD123-expressing malignancies, such as Acute Myeloid Leukemia (AML), Myelodysplastic Syndromes (MDS) and Acute Lymphoblastic Leukemia (ALL). CD123 is the interleukin-3 (IL-3) receptor alpha chain and is frequently over-expressed on the surface of leukemic blasts, as well as leukemic stem cells. CD3 is a protein that is expressed on the surface of T cells and is an essential T cell activating molecule required to induce T cell-mediated cytotoxicity. In preclinical studies, IGM-2537 demonstrated potent T cell-mediated cytotoxicity against leukemia cells but less cytokine release compared to a bispecific IgG. The company expects to initiate the first clinical trial of IGM-2537 in 2024.
Third-Party Agreements
Sanofi Collaboration and License Agreement
In March 2022, the company entered into a global collaboration and license agreement with Genzyme Corporation (Sanofi Agreement), a wholly owned subsidiary of Sanofi (Sanofi), pursuant to which the company will collaborate with Sanofi to generate, develop, manufacture and commercialize IgM antibodies directed to six primary targets, three of which are intended as oncology targets and three of which are intended as immunology targets (Collaboration Targets). The Sanofi Agreement became effective in May 2022.
Medivir Agreement
In January 2021, the company entered into an exclusive license agreement with Medivir AB (Medivir) through which the company received global, exclusive development and commercialization rights for birinapant, a clinical-stage Second Mitochondrial-derived Activator of Caspases (SMAC) mimetic.
Competition
With respect to IGM-8444, the company is aware of other companies with competing clinical stage therapeutics that target DR5 that include, but are not limited to, AbbVie, Beijing Sunbio Biotech, Boehringer Ingelheim, Clover Biopharmaceuticals, Daiichi Sankyo, and InhibRx.
With respect to imvotamab, the company is aware of other companies with competing clinical stage therapeutics that target CD20 that include, but are not limited to, Genmab/AbbVie, Regeneron, Roche/Genentech, and Xencor/Janssen.
With respect to IGM-7354, the company is aware of other companies with competing clinical stage therapeutics that utilize targeted and untargeted interleukin-15 (IL-15) that include, but are not limited to, ImmunityBio, Kadmon/Sanofi, Nektar, Roche/Genentech, SOTIO Biotech and Pfizer.
With respect to IGM-2644, the company is aware of other companies with competing products or product candidates that target CD38 that include, but are not limited to, Genmab, Ichnos Sciences, I-Mab, Janssen, MorphoSys, Sanofi, and Xencor.
With respect to IGM-2537, the company is aware of other companies with competing products or product candidates that target CD123 that include, but are not limited to, Aptevo Therapeutics, Immunogen, Johnson & Johnson, Macrogenics, Menarini Group, Sanofi, and Xencor.
Intellectual Property
As of December 31, 2022, the company’s patent portfolio related to its platform and manufacturing technologies included 14 patent families (13 wholly owned, one exclusively licensed) and included issued U.S. and international patents directed to the company’s modified J chain technology. The platform and manufacturing portfolio includes 37 granted patents, three allowed applications, and 98 pending applications in active prosecution in 16 countries or regions. These patent families are projected to expire between 2034 and 2043, absent any patent term adjustments or extensions. Summaries of relevant published patent families are provided as follows.
The Modified J Chain family includes disclosure and claims related to IgM, IgA, and hybrid multimeric antibodies that include a J chain, where the J chain has been modified to include a binding moiety, e.g., an antibody or antibody fragment, or any other protein or non-protein moiety that can bind to a cognate binding partner (including antibody drug conjugates). The application family also includes disclosure and claims related to methods of making and using multimeric antibody molecules comprising a modified J chain, e.g., bispecific IgM antibodies. This patent family has a projected expiration date of April 2, 2035, absent any patent term adjustments or extensions. The Modified J Chain patent family includes granted patents in the United States (three patents), Australia (two patents), China, Europe (two patents, both validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Hong Kong, Hungary, Ireland, Italy, Luxembourg, the Netherlands, Norway, Poland, Portugal, Sweden, Slovenia, and the United Kingdom, one additionally validated in the Czech Republic and Turkey), Israel (two patents), India, Japan (two patents), Mexico (two patents), Russia, Singapore, South Africa, and South Korea, and is allowed in the United States and New Zealand. As of December 31, 2022, the patent family also included pending patent applications in the United States, Australia, Brazil, Canada, China, Europe, Israel, Russia, Singapore, South Africa, and South Korea. The granted U.S., European, and Chinese claims are directed to IgM antibodies (in the first patents in the United States and Europe); IgM, IgA and hybrid antibodies (in India, Russia, Singapore, and South Korea; in the first patent in Australia; in the second patents in United States and Europe; and in the first and second patents in Israel, Japan, and Mexico); and polymeric antibodies (in South Africa, in the third United States patent, and in the second patent in Australia) comprising a modified J chain with a binding moiety fused or chemically conjugated to selected regions of the J chain. Related claims are being prosecuted in the pending applications.
Two later-filed patent families are related to the company’s Modified J Chain family. These two patent families both have a projected expiration date of September 30, 2036, absent any patent term adjustments or extensions. Patent applications in the first of these two families includes disclosure and claims related to multimeric antibodies (e.g., IgM, IgA, or hybrid multimeric antibodies) that include a modified J chain, where the modified J chain includes a binding moiety that modulates a T cell inhibitory pathway, e.g., CTLA4, PD-1, TIM3, LAG3, BTLA, VISTA or TIGIT. This family includes a granted patent in Europe (validated in Belgium, Denmark, Finland, France, Germany, Ireland, Italy, Luxembourg, the Netherlands, Norway, Spain, Sweden, Switzerland, and the United Kingdom) and Japan. One patent application in this family is pending in the United States. Patent applications in the second of these two families includes disclosure and claims related to multimeric antibodies (e.g., IgM, IgA, or hybrid multimeric antibodies) that include a modified J chain, where the modified J chain includes a moiety that affects adsorption, distribution, metabolism, and/or excretion (ADME) of the multimeric antibody. Exemplary moiety types include, but are not limited to, proteins that increase antibody serum half-life, proteins that affect receptor-mediated transcytosis, and proteins that increase retention of the multimeric antibody in an extravascular space. This patent family also supports product claims covering imvotamab. Patents with claims covering imvotamab are granted in the United States, Japan and Australia and a patent application with claims covering multimeric antibodies comprising a modified J chain comprising albumin is allowed in the United States. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe (two applications), and Japan.
The company’s platform and manufacturing technology portfolio also includes a patent family with disclosure and claims related to J chain and IgM Fc mutations that inhibit binding of IgM to certain multimeric Ig receptors, including the Fcaµ receptor, the Fcµ receptor, and the polymeric Ig receptor. The claims are related to IgM and IgM-derived antibodies that include these mutations, and have substantially increased serum half-lives relative to wild type IgM antibodies. The patent applications in this family have a projected expiration date of March 1, 2039, absent any patent term adjustments or extensions. This family includes a granted patent in the United States. The family includes pending applications in the United States, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, New Zealand, and Singapore.
The company’s platform and manufacturing technology portfolio also includes a patent family that includes disclosure and claims related to IgM antibody Fc modifications that affect the ability of the IgM antibody to trigger complement-dependent cytotoxicity (CDC). Patent applications in this family disclose and claim single and combined human IgM Fc amino acid substitutions that reduce and/or completely inhibit IgM’s typical CDC activity. Applications in this patent family have a projected expiration date of April 6, 2038, absent any patent term adjustments or extensions. This family includes a granted patent in the United States. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, New Zealand, and Singapore.
The company’s platform and manufacturing technology portfolio includes a patent family that includes disclosure and claims related to multimeric molecules with non-antibody moieties on an IgM- or an IgA-based scaffold. For example, applications in this patent family cover IgM or IgA based fusion proteins that include, e.g., ligands, soluble portions of receptors, or cytokines. An exemplary molecule includes an IgM-based scaffold where the IgM heavy chain constant regions are fused to a soluble portion of PD-L1. Applications in this patent family have a projected expiration date of October 23, 2039, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, New Zealand, and Singapore.
The company’s platform and manufacturing technology portfolio also includes a patent family that discloses and claims highly sialylated multimeric binding molecules and methods of making the same. Applications in this family have a projected expiration date of January 5, 2041, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, Malaysia, New Zealand, Singapore and South Africa.
The company’s platform and manufacturing technology portfolio also includes a patent family that discloses and claims multimeric IgM antibodies comprising modifications that reduce asparagine-linked glycosylation. Applications in this family have a projected expiration date of August 21, 2040, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, New Zealand, and Singapore.
The company’s platform and manufacturing technology portfolio also includes a patent family that discloses and claims multimeric antibodies with enhanced selectivity for cells with high target density. Applications in this family have a projected expiration date of September 18, 2040, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe, Israel, Japan, New Zealand, and Singapore.
Product Candidates and Discovery Pipeline
The company’s product candidates and discovery pipeline patent portfolio includes 24 patent families (21 families are wholly owned and three families are exclusively licensed; one family in common with the platform portfolio), including claims directed to its product candidates. These include six patent families with claims directed to imvotamab (three published); and four patent families with claims directed to the company’s DR5 IgM antibody product candidates, including IGM-8444 (three published). The company’s wholly owned product and discovery pipeline portfolio also includes granted patents in the United States, Europe, and Israel with claims directed to IgM antibody superagonists specific for TNFrSF targets. As of December 31, 2022, the company’s product and discovery pipeline portfolio included 82 granted patents (27 wholly owned and 55 exclusively licensed), 106 applications in active prosecution (103 wholly owned and 3 exclusively licensed) in 17 countries or regions, four allowed applications (wholly owned), four pending Patent Cooperation Treaty (PCT) applications (three published; all wholly owned) and 14 unpublished pending U.S. provisional applications covering nine families (13 wholly owned and one exclusively licensed). These patent families are projected to expire between 2036 and 2043, absent any patent term adjustments or extensions. The company wholly owns the rights to these patent families. Summaries of published patent families relevant to the company’s product candidates and its discovery pipeline are provided as follows.
Three wholly owned published patent families with claims directed to imvotamab have projected expiration dates of March 4, 2036, September 30, 2036, and November 17, 2041, respectively, absent any patent term adjustments or extensions. The first patent family includes claims directed to multimeric antibodies, e.g., IgM and IgA antibodies, that include the imvotamab CD20 antigen binding domains and methods of treating cancer patients with such antibodies. This patent family further discloses antibodies that include a modified J chain, where the modified J chain includes an antigen-binding domain specific for CD3-epsilon. This patent family includes claims that encompass the imvotamab composition, as well as methods of making and using the same. Patents with claims that cover imvotamab are granted in the United States, Australia, Brazil, China, Europe (validated in Austria, Belgium, the Czech Republic, Denmark, Finland, France, Germany, Greece, Hong Kong, Hungary, Iceland, Ireland, Italy, Luxembourg, the Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden, Switzerland, Turkey, and the United Kingdom), Japan, South Korea, and Singapore, and a patent application is allowed in Israel. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe, India, Israel, Japan, South Korea, New Zealand, and Singapore. The second family (the ADME family referred to above under platform applications), includes claims directed to multimeric, e.g., IgM and IgA antibodies, that include the imvotamab CD20 antigen binding domains and a modified J chain, where the modified J chain is fused to an antigen-binding domain specific for CD3-epsilon and also to human serum albumin (HSA). The application family also includes claims to methods of making and using the claimed antibodies. Patents with claims covering imvotamab are granted in the United States, Japan and Australia and a patent application with claims covering multimeric antibodies comprising a modified J chain comprising albumin is allowed in the United States. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe, and Japan. The third family has an international patent application, filed under that PCT that discloses and claims various methods of treatment with imvotamab, including specific dosing regimens. Patents granting from national stage applications filed from this PCT application will have a projected expiration date of November 17, 2041, absent any patent term adjustments or extensions The PCT application is in the international stage and will enter national stage prosecution on or before 17 May 2023 or 17 June 2023, depending on the jurisdiction.
The company’s patent portfolio also includes four patent families owned by it directed to its TNFrSF superagonist technology and product candidates. The first patent family includes disclosure and claims directed to multimeric superagonist antibodies that bind to any TNFrSF target. This family also includes disclosure and claims directed multimeric superagonist antibodies that bind to DR5 that relate to the company’s DR5 IgM antibody product candidates. The application family, which the company wholly own, has a projected expiration date of January 20, 2036, absent any patent term adjustments or extensions, and includes two granted U.S. patents, as well as granted patents in Europe (validated in Austria, Belgium, the Czech Republic, Denmark, Finland, France, Germany, Hong Kong, Hungary, Iceland, Ireland, Italy, Luxembourg, the Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden, Switzerland, Turkey, and the United Kingdom), Australia (two patents), Israel, Japan, South Korea, New Zealand, and Singapore. The claims in the first granted U.S. patent, as well as the Australian patent are generically directed to IgM-based TNFrSF superagonists and their use in treating cancer patients. The European and Israeli patents contain similar claims, as well as claims that relate to the company’s DR5 IgM product candidates, including IGM-8444. The claims in the second granted U.S. Patent and the granted patents in Japan, South Korea, New Zealand, and Singapore are directed to DR5 IgM product candidates, including IGM-8444. The patent family is also pending in Canada, China, Europe, India, Israel, Japan, New Zealand, and Singapore, with claims relating broadly to TNFrSF superagonists and also to DR5 superagonists. Claims directed to polynucleotides encoding the company’s DR5 IgM product candidates are allowed in the United States.
The company’s patent portfolio includes a wholly owned patent family directed to a specific TNFrSF target, CD40. The CD40 family has a projected expiration date of July 19, 2037, absent any patent term adjustments or extensions, and includes claims directed to a variety of different multimeric CD40 superagonist antibodies and their use for treating cancer patients. Patent applications in this family are pending in the United States, Canada, and Europe and a patent application is allowed in Australia.
The company’s patent portfolio includes a wholly owned patent family directed to combination cancer therapies that include a DR5 superagonist antibody, e.g., the company’s DR5 IgM antibody product candidates, including IGM-8444, in combination with a chemotherapeutic agent, e.g., irinotecan, gemcitabine, or Venetoclax. Patent applications in this family are pending in the United States, Australia, Canada, China, Europe, Japan, and South Korea. This family has a projected expiration date of February 25, 2039, absent any patent term adjustments or extensions.
The company’s patent portfolio also includes a wholly owned patent family directed to combination cancer therapies that include a DR5 superagonist antibody, e.g., the company’s DR5 IgM antibody product candidates, including IGM-8444, in combination with a cancer therapy, e.g., birinapant, oxaliplatin, carboplatin, paclitaxel, or radiation. Patent applications in this family have a projected expiration date of May 12, 2041, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, Malaysia, New Zealand, Singapore, and South Africa.
The company’s patent portfolio also includes three patent families exclusively licensed from Medivir AB that are related to small molecule SMAC mimetics, e.g., birinapant. The projected expiration dates of the first, second, and third families are July 15, 2025, February 27, 2026, and June 25, 2030, respectively, absent any patent term adjustments or extensions. The first patent family includes three granted U.S. patents, as well as granted patents in Europe (validated in France, Germany, Italy, Spain, and the United Kingdom), Australia, Canada, Japan, and Mexico. The second patent family includes six granted U.S. patents, as well as granted patents in Europe (validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Hong Kong, Hungary, Ireland, Italy, the Netherlands, Sweden, and the United Kingdom), Australia, Canada, China, Eurasia (validated in Russia), Israel, India, Japan, South Korea, Mexico, Singapore, and South Africa. The third patent family includes claims directed to the chemical species of birinapant and includes seven granted U.S. patents, as well as granted patents in Europe (validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Hong Kong, Hungary, Ireland, Italy, the Netherlands, Sweden, and the United Kingdom), Australia, Brazil, Canada, Chile, China, Colombia, Eurasia (validated in Russia), Israel, India, Japan, South Korea, Mexico, Malaysia, New Zealand, Peru, the Philippines, Taiwan, Ukraine, and South Africa. Patent applications in the third family are pending in the United States, Thailand, and Venezuela.
The company’s patent portfolio also includes four patent families (two published) related to IGM-7354, its IL-15 x PD-L1 bispecific IgM antibody. The first patent family is directed to the identification and characterization of novel PD-L1 antibodies. This application family, titled Anti-PD-L1 Antibodies, has a projected expiration date of May 9, 2037, absent any patent term adjustments or extensions and includes granted patents in the United States, Europe (validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Hong Kong, Hungary, Ireland, Italy, Luxemburg, the Netherlands, Norway, Poland, Portugal, Sweden, and the United Kingdom), China, Japan, South Korea, and Singapore. Patent applications in this family are pending in the United States, Australia, Canada, India, Israel, and New Zealand. The second patent family is wholly owned and is directed to the multimeric binding molecules comprising a J chain comprising an immunostimulatory agent, e.g., IL-15, and the claims cover the company’s product candidate IGM-7354. Patent applications in this family have a projected expiration date of August 14, 2040, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, New Zealand, and Singapore.
The company’s patent portfolio also includes two families related to IGM-2537, its CD123 x CD3 bispecific IgM antibody. The first patent family is directed to multimeric CD123 x CD3 bispecific antibody. This application family, titled Multimeric Bispecific Anti-CD123 Binding Molecules and Uses Thereof, has a projected expiration date of August 14, 2040, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Australia, Brazil, Canada, China, Europe, Israel, India, Japan, South Korea, Mexico, New Zealand, and Singapore. The second patent family includes a wholly owned international patent application, filed under the PCT that is directed to novel humanized CD123 antibodies. Patents granting from national stage applications filed from this PCT application will have a projected expiration date of February 17, 2042, absent any patent term adjustments or extensions.
The company’s patent portfolio also includes an unpublished patent family related to IGM-2644, or CD38 x CD3 bispecific IgM antibody. Applications in this family have a projected expiration date of February 3, 2042, absent any patent term adjustments of extensions.
The company’s patent portfolio also includes two patent families (one published) related to viral trapping of coronaviruses (including SARS-CoV2, the virus that causes Covid 19) that utilize the human ACE2 receptor for entry into cells. The first patent family, titled Multimeric Coronavirus Binding Molecules and uses thereof, has a projected expiration date of July 27, 2041, absent any patent term adjustments or extensions. Patent applications in this family are pending in the United States, Europe, Canada, and Australia. The second unpublished application has a projected expiration date of August 8, 2043, absent any patent term adjustments or extensions.
Research and Development
For the year ended December 31, 2022, the company’s research and development expenses were $179.3 million.
Government Regulation
The Food and Drug Administration (FDA) and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of biologics, such as those the company is developing. Prior to beginning the first clinical trial with a product candidate, the company must submit an IND to the FDA.
History
The company was incorporated in the state of Delaware in 1993. It was formerly known as Palingen, Inc. and changed its name to IGM Biosciences, Inc. in 2010.
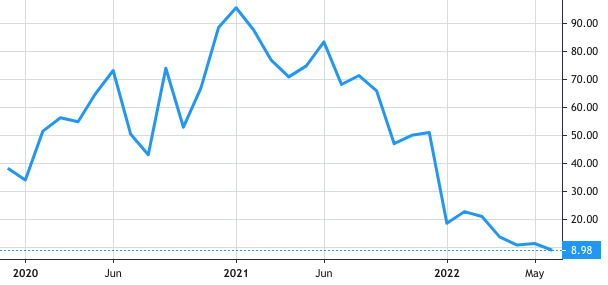
 Stock Value
Stock Value

