About ICU Medical
ICU Medical, Inc. (ICU) develops, manufactures, and sells innovative medical products used in infusion therapy, vascular access, and vital care applications. The company focuses on providing quality, innovation and value to its clinical customers worldwide. ICU's product portfolio includes ambulatory, syringe, and large volume IV pumps and safety software; dedicated and non-dedicated IV sets, needlefree IV connectors, IV catheters, sharps safety products, and sterile IV solutions; closed system transfer devices and pharmacy compounding systems; as well as a range of respiratory, anesthesia, patient monitoring, and temperature management products.
The company’s primary customers are acute care hospitals, wholesalers, ambulatory clinics and alternate site facilities, such as outpatient clinics, home health care providers, and long-term care facilities. The company has grown organically and through acquisition.
Products
The company’s business units include Consumables, Infusion Systems and Vital Care.
Consumables
Consumables business unit includes Infusion Therapy, Oncology, Vascular Access and Tracheostomy products.

Infusion Therapy
Infusion Therapy products include non-dedicated infusion sets, extension sets, needle-free connectors, and disinfection caps. Infusion sets used in hospitals and ambulatory clinics consist of flexible sterile tubing running from an IV bag or bottle containing a drug product or solution to a catheter inserted in a patient’s vein that may or may not be used with an infusion pump. Disinfection caps are used to actively disinfect access points into the infusion sets and catheters. The company’s primary Infusion Therapy products are:
Clave needlefree products, including the MicroClave, MicroClave Clear, and NanoClave brand of connectors, accessories, extension and administration sets used for the administration of IV fluids and medications;
Neutron catheter patency device, used to help maintain patency of central venous catheters;
Tego needlefree connector utilized to access catheters for hemodialysis and apheresis applications; and
ClearGuard, SwabCap and SwabTip disinfection caps.
Oncology
Closed System Transfer Devices (CSTD) and hazardous drug compounding systems are used to prepare and deliver hazardous IV medications, such as those used in chemotherapy, which, if released, can have harmful effects on the healthcare worker and environment. The company’s primary Oncology products are:
ChemoLock CSTD, which utilizes a proprietary needlefree connection method, is used for the preparation and administration of hazardous drugs. ChemoLock is used to limit the escape of hazardous drug or vapor concentrations, block the transfer of environmental contaminants into the system, and eliminates the risk of needlestick injury;
ChemoClave, an ISO Connection standard and universally compatible CSTD used for the preparation and administration of hazardous drugs. ChemoClave utilizes standard ISO luer locking connections, making it compatible with all brands of needlefree connectors and pump delivery systems. ChemoClave also is used to limit the escape of hazardous drug or vapor concentrations, block the transfer of environmental contaminants into the system, and eliminate the risk of needlestick injury; and
Deltec GRIPPER non-coring needles for portal access.
The preparation of hazardous drugs typically takes place in a pharmacy where drugs are removed from vials and prepared for delivery to a patient. Those prepared drugs are then transferred to a nursing unit where the chemotherapy is administered via an infusion pump set to a patient. Components of the ChemoClave and ChemoLock product lines are used both in pharmacies and on the nursing floors for the preparation and administration of hazardous drugs.
Vascular Access
The company’s Vascular Access products are used by clinicians to access the patients' bloodstream to deliver fluids and medication or to obtain blood samples. The company’s primary Vascular Access products are Jelco safety and conventional peripheral IV catheters and sharps safety devices for hypodermic injection, designed to help prevent accidental needlestick injury; Safe-T Wing venipuncture and blood collection devices; Port-A-Cath implantable ports; Portex arterial blood sampling syringes; PowerWand midline catheters; and Cleo subcutaneous infusion catheters and sets.
Tracheostomy
The company’s tracheostomy products are used in the placement of a secure airway using both surgical and percutaneous insertion techniques. The company’s primary Tracheostomy products includes:
Portex BLUselect PVC tracheostomy tubes, which feature an inner cannula, as well as a Suctionaid option for above the cuff suctioning and vocalization capability;
Portex Bivona silicone tracheostomy tubes, which offer the added benefits of comfort and mobility and come in a variety of configurations suited to meet the clinical needs of neonatal through adult patients; and
Portex BLUperc percutaneous insertion kits, which allow for safe placement of the tracheostomy tube at the bedside.
Infusion Systems
The company offers a comprehensive portfolio of infusion pumps, dedicated IV sets, software and professional services to meet the wide range of infusion needs. The company’s primary Infusion System products include:
Large Volume Pump (LVP) Hardware:
Plum 360infusion pumps feature a unique delivery system that helps to enhance patient safety and workflow efficiency. The pumps work with PlumSet dedicated IV sets that include an air trap to help minimize interruptions and a direct connection to the secondary line that eliminates the risk of setup errors and enables concurrent delivery of two compatible medications through a single line. Plum 360 has been named Best in KLAS for seven years in a row (2018, 2019, 2020, 2023 – Best in KLAS Smart Pump Traditional; 2021, 2022, 2023, 2024 Best in KLAS Smart Pump EMR Integrated) and was the first medical device to be awarded UL Cybersecurity Assurance Program Certification.
Plum Duo infusion pumps with LifeShield safety software are dual channel devices capable of delivering up to four compatible medications at independent rates with a single pump. The Plum Duo combines the award-winning legacy of Plum 360 with modern innovation, including a large touch screen and highly intuitive user interface to help guide users through programming, while streamlining complex tasks.
Ambulatory Infusion Hardware:
CADD ambulatory infusion pumps and disposables, including administration sets and medication cassette reservoirs, serve as a single pain management platform across all types of IV pain management therapies and all clinical care areas from the hospital to outpatient treatment.
Syringe Infusion Hardware:
Medfusion syringe infusion pumps are designed for the administration of fluids and medication to address the needs of the most vulnerable patients requiring precisely controlled infusion rates. Focused on delivery accuracy, the Medfusion 4000 can deliver from a comprehensive portfolio of syringes to meet syringe pump guidance to deliver medication from the smallest syringe size possible.
IV Medication Safety Software:
ICU Medical MedNet software is an enterprise-class medication management platform that can help reduce medication errors, improve quality of care, streamline workflows and maximize revenue capture. ICU Medical MedNet connects its industry-leading Plum 360 smart pumps to a hospital’s EHR, asset tracking systems, and alarm notification platforms to further enhance infusion safety and efficiency.
LifeShield infusion safety software for Plum Duo infusion pumps is an enterprise-wide platform designed with the input of pharmacists, nurses and administrators to empower health systems to raise the bar in IV performance. The system’s hybrid architecture provides cloud-based functionality to allowing access anywhere with on-premise management providing security and control.
PharmGuard medication safety software for Medfusion 4000 syringe and CADD-Solis pumps allows for customized drug libraries to support the standardization of protocols for medication administration throughout the facility.
Professional Services
In addition to the products, the company’s teams of clinical and technical experts work with customers to develop safe and efficient infusion systems, providing customized and personalized configuration, implementation, and data analytics services to optimize its infusion hardware and software.
Vital Care
The company’s Vital Care business unit includes IV Solutions, Hemodynamic Monitoring, General Anesthesia and Respiratory, Temperature Management Solutions and Regional Anesthesia/Pain Management products.
IV Solutions
The company’s IV Solutions products include a broad portfolio of injection, irrigation, nutrition and specialty IV solutions including:
IV Therapy and Diluents, including Sodium Chloride, Dextrose, Balanced Electrolyte Solutions, Lactated Ringer's, Ringer's, Mannitol, Sodium Chloride/Dextrose and Sterile Water.
Irrigation, including Sodium Chloride Irrigation, Sterile Water Irrigation, Physiologic Solutions, Ringer's Irrigation, Acetic Acid Irrigation, Glycine Irrigation, Sorbitol-Mannitol Irrigation, Flexible Containers and Pour Bottle Options.
Hemodynamic Monitoring
The company’s Hemodynamic Monitoring products are designed to help clinicians get accurate real-time access to patients’ hemodynamic and cardiac status with an extensive portfolio of monitoring systems and advanced sensors and catheters. Measurements provided by the company’s systems help clinicians determine how well the heart is pumping blood and how efficiently oxygen from the blood is being used by the tissues. The company’s Hemodynamic Monitoring products include Cogent 2-in-1 hemodynamic monitoring system; CardioFlo hemodynamic monitoring system; TDQ and OptiQ cardiac output monitoring catheters; TriOx venous oximetry catheters; Transpac blood pressure transducers; SafeSet closed blood sampling and conservation system; and MEDEX LogiCal Pressure Monitoring System and components.
General Anesthesia & Respiratory
The company offers a broad range of anesthesia systems and devices and breathing circuits, ventilation, respiratory and specialty airway products that maintain patients’ airways before, during and after surgery. The company’s primary Anesthesia & Respiratory products are:
Portex acapella bronchial hygiene products used to mobilize pulmonary secretions to facilitate the opening of airways in patients with chronic respiratory diseases such as chronic obstructive pulmonary disease, or COPD, asthma and cystic fibrosis.
Temperature Management Solutions
Temperature Management solutions systems are used in perioperative and critical care settings to help monitor and regulate patient temperature. The company’s primary Temperature Management products include:
Level 1 rapid infusion, fluid warming, routine blood and fluid warming, irrigation fluid warming, convective patient warming and temperature probes.
Regional Anesthesia/Pain Management Trays
The company offers a comprehensive range of Portex regional anesthesia/pain management trays and components. The company’s primary products include Epidural Trays; Spinal Trays; Combined (CSE) Trays; Peripheral Nerve Block Trays; and Specialty Trays (Lumbar Puncture, Amniocentesis, Myelogram).
Sales, Marketing and Administration
The company sells globally through its own direct sales force and through independent distributors. The company serves customers in over 100 countries throughout the world. The majority of the company’s sales is denominated in United States (U.S.) dollars and it has sales denominated in Euros, Canadian dollars, Japanese Yen, British Pound and Australian dollars, as well as other currencies. In 2023, the company had worldwide net sales to Medline of 16% of consolidated net sales.
Distribution
The company’s products are marketed and distributed in the U.S. and internationally to medical product manufacturers, independent distributors and directly to end users. The distribution of the company’s products in the U.S. is supported by a network of owned and leased distribution centers, which include King of Prussia, Pennsylvania; Los Angeles, California; Dallas, Texas and Olive Branch, Mississippi. The company also utilizes a number of public warehouses as part of its supply chain. Internationally, the company manages distribution by utilizing international regional hubs and through independent distributors.
Research and Development
The company’s research and development costs were $85.3 million in 2023.
Competition
Consumables: The company competes with products and systems marketed by Becton Dickinson (BD), Baxter International (Baxter), B. Braun Medical, Inc. (B. Braun), Angiodynamics and Teleflex.
Infusion Systems: The company faces strong global competitors in the Infusion Systems market. In the United States (U.S.), the company’s competitors include BD, Baxter, B. Braun, Moog Medical, and Fresenius Kabi, a division of Fresenius Group. Outside of the U.S., the company’s primary competitors are BD, B. Braun, Fresenius, and a large number of local market pump manufacturers.
Vital Care: The company’s IV Solutions products are sold in the U.S. and Canada and compete in the U.S. with Baxter and B. Braun. The company’s primary competitors include Edwards Lifesciences, Belmont and Intersurgical plc.
Government Regulation
The company’s products and operations are subject to extensive and rigorous regulation by the Food and Drug Administration (FDA) and other federal, state and local authorities, as well as foreign regulatory authorities. The Federal Trade Commission (FTC) also regulates the advertising of the company’s products.
The majority of the company’s products are regulated by the FDA as medical devices in the U.S. Unless an exemption applies, each new or significantly modified medical device the company seeks to commercially distribute in the U.S. will require either a pre-market notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDC Act, also referred to as a 510(k) clearance, or approval from the FDA of a pre-market approval (PMA) application. The company must also comply with FDA and International Organization for Standardization (ISO) governing medical device manufacturing practices. The company is a FDA and International Organization for Standardization (ISO) registered medical device manufacturer, and must demonstrate that it and its contract manufacturers comply with the FDA's Quality System Regulation (QSR), cGMPs (current good manufacturing practices) and similar foreign requirements.
The company is subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which it conducts its business. These laws include the federal Anti-Kickback Statute; federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other federal third-party payors that are false or fraudulent; the federal Civil Monetary Penalties Law; the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act; and the federal Physician Payment Sunshine Act.
History
ICU Medical, Inc. was founded in 1984. The company was incorporated in 1992.

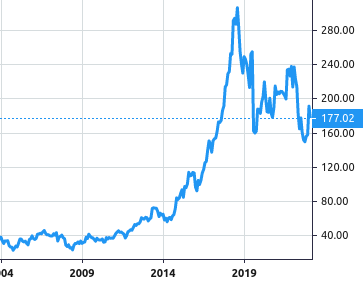
 Stock Value
Stock Value

