About Henry Schein
Henry Schein, Inc. (Henry Schein) operates as a solutions company for health care professionals powered by a network of people and technology.
The company is the world's largest provider of health care products and services primarily to office-based dental and medical practitioners, as well as alternate sites of care.
With experience distributing health care products, the company has built a vast set of small, mid-sized and large customers in the dental and medical markets, serving more than one million customers worldwide across dental practices, laboratories, physician practices, and ambulatory surgery centers, as well as government, institutional health care clinics and other alternate care clinics.
The company stocks a comprehensive selection of more than 300,000 branded products and Henry Schein corporate brand products through the company's main distribution centers. The company's infrastructure, including over 5.3 million square feet of space in 36 strategically located distribution and 22 manufacturing facilities around the world, enables the company to historically provide rapid and accurate order fulfillment, and better serve the company's customers.
Segments
The company conducts its business through two reportable segments: Health Care Distribution and Technology and Value-Added Services. These segments offer different products and services to the same customer base.

The company's dental businesses serve office-based dental practitioners, dental laboratories, schools, government and other institutions. The company's medical businesses serve physician offices, urgent care centers, ambulatory care sites, emergency medical technicians, dialysis centers, home health, federal and state governments and large enterprises, such as group practices and integrated delivery networks, among other providers across a wide range of specialties.
The Health Care Distribution reportable segment, combining the company's global dental and medical operating segments, distributes consumable products, small equipment, laboratory products, large equipment, equipment repair services, branded and generic pharmaceuticals, vaccines, surgical products, dental specialty products (including implant, orthodontic and endodontic products), diagnostic tests, infection-control products, personal protective equipment products ('PPE') and vitamins. While the company's primary go-to-market strategy is in the company's capacity as a distributor, the company also markets and sells under its own corporate brand portfolio of high-quality consumable merchandise products, and manufactures certain dental specialty products in the areas of oral surgery, implants, orthodontics and endodontics.
The Technology and Value-Added Services reportable segment provides software, technology and other value-added services to health care practitioners. Henry Schein One, the largest contributor of sales to this category, offers dental practice management solutions for dental and medical practitioners. In addition, the company offers dentists and physicians a broad suite of electronic health records, patient communication services, including electronic marketing and website design, analytics and patient demand generation. The company's value-added practice solutions include practice consultancy, education, integrated revenue cycle management and the facilitation of financial service offerings (on a non-recourse basis) to help dentists and physicians operate and expand their business operations, e-services, practice technology, network and hardware services, as well as consulting, and continuing education services for practitioners. The company's hands-on consultative approach to provide solutions to support practice decision-making is a key differentiator for the company's business.
Business Strategy
The company's BOLD+1 Strategic Plan consists of the following:
Build ('B'): Complementary software, specialty, and services businesses for high growth.
Operationalize ('O'): One Distribution to deliver exceptional customer experience, increased efficiency, and growth.
Leverage ('L'): One Schein to broaden and deepen relationships with the company's customers.
Drive ('D'): Drive digital transformation for the company's customers and for Henry Schein.
+1: Create Value for the company's stakeholders.
The company's strategies are to increase penetration of the company's existing customer base; increase the number of customers the company serves; leverage the company's value-added products and services; and pursue strategic acquisitions and joint ventures.
Markets Served
The company supports its dental and medical professionals through the many SKUs that the company offers, as well as through important value-added services, including practice management software, electronic claims processing, financial services and continuing education, all designed to help maximize a practitioner's efficiency.
Governmental Regulations
Certain of the company's businesses are subject to local, state and federal governmental laws and regulations relating to the distribution of pharmaceuticals and medical devices and supplies. Among the United States federal laws applicable to the company is the Controlled Substances Act, the Federal Food, Drug, and Cosmetic Act, as amended ('FDC Act'), Section 361 of the Public Health Service Act and Section 401 of the Consolidated Appropriations Act of the Social Security Act, as well as laws regulating the billing of and reimbursement from government programs, such as Medicare and Medicaid, and from commercial payers. The company is also subject to comparable foreign regulations.
In addition, with respect to the company's specialty home medical supply business, the company is subject to certain state licensure laws (including state pharmacy laws), and also certain accreditation standards, including to qualify for reimbursement from Medicare and other third-party payers.
As a distributor of controlled substances, the company is required, under the Controlled Substances Act, to obtain and renew annually registrations for the company's facilities from the United States Drug Enforcement Administration ('DEA') permitting the company to handle controlled substances. The company is also subject to other statutory and regulatory requirements relating to the storage, sale, marketing, handling, reporting, record-keeping and distribution of such drugs, in accordance with the Controlled Substances Act and its implementing regulations, and these requirements have been subject to heightened enforcement activity in recent times. The company is subject to inspection by the DEA. Certain of the company's businesses are also required to register for permits and/or licenses with, and comply with operating and security standards of, the DEA, the FDA, the United States Department of Health and Human Services ('HHS'), and various state boards of pharmacy, state health departments and/or comparable state agencies, as well as comparable foreign agencies, and certain accrediting bodies, depending on the type of operations and location of product distribution, manufacturing or sale. These businesses include those that distribute, manufacture, relabel, and/or repackage prescription pharmaceuticals and/or medical devices and/or HCT/P products, or own pharmacy operations, or install, maintain or repair equipment.
In addition, Section 301 of the National Organ Transplant Act, and a number of comparable state laws, impose civil and/or criminal penalties for the transfer of human organs, as defined in the regulations, for valuable consideration, while generally permitting payments for the reasonable costs incurred in their procurement, processing, storage and distribution. The company is also subject to foreign government regulation of such products. The DEA, the FDA and state regulatory authorities have broad inspection and enforcement powers, including the ability to suspend or limit the distribution of products by the company's distribution centers, seize or order the recall of products and impose significant criminal, civil and administrative sanctions for violations of these laws and regulations. Foreign regulations subject the company to similar foreign enforcement powers.
Certain of the company's businesses are subject to federal and state (and similar foreign) health care fraud and abuse, referral and reimbursement laws and regulations with respect to their operations. Some of these laws, referred to as 'false claims laws,' prohibit the submission or causing the submission of false or fraudulent claims for reimbursement to federal, state and other health care payers and programs.
The company is directly or indirectly subject to numerous and evolving federal, state, local and foreign laws and regulations that protect the privacy and security of personal information, such as the federal Health Insurance Portability and Accountability Act of 1996,as amended, and implementing regulations ('HIPAA'), the Controlling the Assault of Non-Solicited Pornography and Marketing Act ('CAN-SPAM'), the Telephone Consumer Protection Act of 1991 ('TCPA'), Section 5 of the Federal Trade Commission Act ('FTC Act'), the California Privacy Act ('CCPA'), and the California Privacy Rights Act ('CPRA') that became effective on January 1, 2023.
Also, the European Parliament and the Council of the EU adopted the pan-European General Data Protection Regulation ('GDPR'), effective from May 25, 2018, which increased privacy rights for individuals ('Data Subjects'), including individuals who are the company's customers, suppliers and employees.
While the company has substantially compliant programs and controls in place to comply with the GDPR, CCPA, PIPL,CPRA and other state law requirements, the company's compliance with data privacy and cybersecurity laws is likely to impose additional costs on the company.
The company also sells products and services that health care providers, such as physicians and dentists, use to store and manage patient medical or dental records. These customers, and the company, are subject to laws, regulations and industry standards, such as HIPAA and the Payment Card Industry Data Security Standards, which require the protection of the privacy and security of those records.
The company is also subject to certain United States and foreign laws and regulations concerning the conduct of the company's foreign operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, German anti-corruption laws, and other anti-bribery laws and laws pertaining to the accuracy of the company's internal books and records, as well as other types of foreign requirements similar to those imposed in the United States.
Competition
In the dental market, the company's primary competitors in the U.S. are the Patterson Dental division of Patterson Companies, Inc. and Benco Dental Supply Company. The company's primary competitors in the U.S. medical market, which accounts for the large majority of the company's global medical sales, are McKesson Corporation and Medline Industries, Inc., which are national distributors.
With regard to the company's dental software, the company competes against numerous companies, including the Patterson Dental division of Patterson Companies, Inc., Carestream Health, Inc., Carestream Dental LLC, Centaur Software Development Co Pty Ltd. (d.b.a. dental4windows, dental4web), Open Dental Software, Inc., PlanetDDS LLC, Good Methods Global Inc. (d.b.a. CareStack) and Curve Dental, LLC.
In other software end markets, including revenue cycle management, patient relationship management and patient demand generation, the company competes with companies, such as Vyne Therapeutics Inc., EDI-Health Group, Inc. (d.b.a. Dental X Change, Inc.), Weave Communications, Inc., and Solutionreach, Inc.
The medical practice management and electronic medical records market is fragmented and the company competes with numerous companies, such as the NextGen division of Quality Systems, Inc., eClinicalWorks, Allscripts Healthcare Solutions, Inc. and Epic Systems Corporation.
The company also faces significant competition internationally, where the company competes on the basis of price and customer service against several large competitors, including the GACD Group, Proclinic SA, Lifco AB, Planmeca Oy and Billericay Dental Supply Co. Ltd.
Proprietary Rights
The company holds trademarks relating to the 'Henry Schein' name and logo, as well as certain other trademarks.
History
Henry Schein, Inc. was founded in 1932. The company was incorporated in 1992.
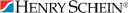
 Stock Value
Stock Value

