About BeiGene
BeiGene, Ltd. operates as a global oncology company discovering and developing innovative treatments that are more accessible and affordable to cancer patients worldwide.
The company has three approved medicines that were internally discovered and developed, including BRUKINSA (zanubrutinib), a small molecule inhibitor of Bruton’s Tyrosine Kinase (‘BTK’) for the treatment of various blood cancers; TEVIMBRA (tislelizumab), an anti-PD-1 antibody immunotherapy for the treatment of various solid tumor and blood cancers; and PARTRUVIX (pamiparib), a selective small molecule inhibitor of PARP1 and PARP2. The company has obtained approvals to market BRUKINSA in the United States (‘U.S.’), the People’s Republic of China (‘China’ or the ‘PRC’), the European Union (‘EU’), the United Kingdom (‘U.K.’), Canada, Australia, and additional international markets; TEVIMBRA (tislelizumab) in the EU and China; and PARTRUVIX in China. By leveraging the company’s strong commercial capabilities, the company has in-licensed the rights to distribute an additional 14 approved medicines for the China market. Supported by the company’s global clinical development and commercial capabilities, the company has entered into collaborations with world-leading biopharmaceutical companies such as Amgen Inc. (‘Amgen’) and Beijing Novartis Pharma Co., Ltd. (‘Novartis’) to develop and commercialize innovative medicines.
The company is committed to advancing best- and first-in-class clinical candidates internally or with like-minded partners to develop impactful and affordable medicines for patients across the globe. Recognizing the importance of clinical trial activities in the company’s industry and the challenges associated with outsourcing to third-party contract research organizations (‘CROs’), the company has built its own 3,000+ person internal clinical team and are largely CRO-free. The company has conducted more than 130 clinical trials in-house, with over 22,000 subjects enrolled in approximately 45 regions. This includes more than 40 pivotal or potentially registration-enabling trials across the company’s portfolio.
The company has built, and is expanding, its internal manufacturing capabilities. The company is building a commercial-stage biologics manufacturing and clinical R&D center at the Princeton West Innovation Park in Hopewell, New Jersey (the ‘Hopewell facility’), in addition to the company’s existing biologic and small molecule manufacturing facilities in China to support demands of the company’s medicines. The company also works with high quality contract manufacturing organizations (‘CMOs’) to manufacture the company’s internally developed clinical and commercial products.
The company is a holding company incorporated in the Cayman Islands with operations primarily conducted through the company’s subsidiaries in the U.S., China, the U.K., Switzerland, and Australia.
Strategy

The company has built a substantial global development and medical affairs team of 3,000+ people on five continents, allowing the company to run clinical trials predominantly without reliance on CROs.
The company has built one of the world’s largest, most productive and cost-effective oncology research teams with 1,100+ scientists.
The company has built a strong commercial portfolio, centered around two foundational medicines, BRUKINSA and TEVIMBRA, that are primary revenue sources and support the development of the company’s future pipeline and additional combination therapies.
The company has a differentiated global commercial organization of over 3,700 people to deliver medicines to patients around the globe, including over 500 in North America and Europe.
Commercial and Registration Stage Products
The company commercialize the following internally developed cancer medicines:
BRUKINSA
BRUKINSA is a next-generation small molecule inhibitor of BTK designed to maximize BTK occupancy and minimize off-target binding effects. It is an orally active inhibitor that covalently binds to BTK, resulting in irreversible inactivation of the enzyme.
The company is marketing BRUKINSA in the U.S., China, Europe, the U.K., Canada, Australia, and other markets.
In the U.S., BRUKINSA received accelerated approval as a treatment for mantle cell lymphoma (‘MCL’) in adult patients who have received at least one prior therapy (November 2019), and has since also been approved for patients with Waldenström’s macroglobulinemia (‘WM’) and R/R marginal zone lymphoma (‘MZL’) who have received at least one anti-CD20-based regimen. The MCL and MZL indications were approved under accelerated approval based on overall response rate. Continued approval for these indications may be contingent upon verification and description of clinical benefit in a confirmatory trial. In January 2023, BRUKINSA was approved by the U.S. FDA for the treatment of adult patients with CLL or SLL. In December 2023, the FDA approved a label update for BRUKINSA to include superior progression-free survival (‘PFS’) results from the Phase 3 ALPINE trial comparing BRUKINSA against IMBRUVICA in previously treated patients with R/R CLL. The FDA is reviewing a supplemental new drug application (‘sNDA’) submission for BRUKINSA in R/R follicular lymphoma (‘FL’) with a Prescription Drug User Free Act date in March 2024.
In Europe, BRUKINSA has received approval from the European Commission (‘EC’) for the treatment of adult patients with WM who have received at least one prior therapy or for the first-line treatment of patients unsuitable for chemo-immunotherapy, as well as for the treatment of patients with R/R MZL and for the treatment of patients with CLL. In November 2023, the EC approved BRUKINSA in combination with obinutuzumab for the treatment of adult patients with R/R FL who have received at least two prior lines of systemic therapy. BRUKINSA is now approved to treat more patient populations in the EU than any other BTK inhibitor.
In China, BRUKINSA has received approval from the China National Medical Products Administration (‘NMPA’) for treatment-naïve adults with CLL or SLL, treatment-naïve adults with WM, R/R CLL/SLL, R/R WM, and conditional approval for adult patients with MCL who have received at least one prior therapy. All five approved indications for BRUKINSA are included in the updated National Reimbursement Drug List (‘NRDL’) by the China National Healthcare Security Administration (‘NHSA’).
BRUKINSA is approved across several indications in more than 65 markets as of February 2024.
TEVIMBRA (tislelizumab)
TEVIMBRA is a humanized IgG4 monoclonal antibody against the immune checkpoint receptor programmed cell death protein 1 (‘PD-1’) that the company specifically designed to minimize binding to Fc receptor gamma, which is believed to play an essential role in activating phagocytosis in macrophages, to minimize its negative impact on T effector cells.
Tislelizumab is approved in China in twelve indications:
Full approval for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma with high PD-L1 expression in combination with fluoropyrimidine and platinum chemotherapy; for first-line treatment of patients with advanced squamous non-small cell lung cancer (‘NSCLC’) in combination with chemotherapy; for first-line treatment of patients with advanced non-squamous NSCLC in combination with chemotherapy; for second- or third-line treatment of patients with locally advanced or metastatic NSCLC who progressed on prior platinum-based chemotherapy; for the treatment of patients with locally advanced or metastatic esophageal squamous cell carcinoma (‘ESCC’) who have disease progression following or are intolerant to first-line standard chemotherapy; for first-line treatment of patients with locally advanced or metastatic ESCC in combination with chemotherapy; for first-line treatment of patients with recurrent or metastatic nasopharyngeal cancer (‘NPC’); for first-line treatment of patients with unresectable or metastatic hepatocellular carcinoma (‘HCC’); for the treatment of patients with HCC who have received at least one systemic therapy; conditional approval for the treatment of patients with classical Hodgkin’s lymphoma (‘cHL’) who received at least two prior therapies; for the treatment of patients with locally advanced or metastatic urothelial carcinoma (‘UC’) with PD-L1 high expression whose disease progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy; and for patients with previously treated, locally advanced unresectable or metastatic microsatellite instability-high (‘MSI-H’) or mismatch repair-deficient (‘dMMR’) solid tumors.
Full approval for these indications is contingent upon results from ongoing randomized, controlled, confirmatory clinical trials.
Tislelizumab was included in the NRDL in 2020 for cHL and UC, in 2021 for non-squamous NSCLC, squamous NSCLC and HCC, in 2022 for locally advanced or metastatic NSCLC, MSI-H solid tumors, locally advanced or metastatic ESCC following progression or intolerance to prior first-line chemotherapy, and first-line recurrent or metastatic NPC, and in 2023 for first-line locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma with high PD-L1 expression and first-line unresectable locally advanced, recurrent or metastatic ESCC.
In addition, three supplemental Biologics License Applications (‘sBLAs’) for tislelizumab for patients with first-line extensive stage small cell lung cancer (‘ES-SCLC’) and for patients with first-line locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma (‘GC/GEJ’) regardless of PD-L1 status and tislelizumab in combination with platinum-based doublet chemotherapy as neoadjuvant treatment for patients with NSCLC are under review by the Center for Drug Evaluation (‘CDE’) of the NMPA.
In Europe, the EC has approved TEVIMBRA as monotherapy for the treatment of adult patients with unresectable, locally advanced or metastatic ESCC after prior platinum-based chemotherapy, along with additional filings.
In the U.S., the company has filed a Biologics License Application (‘BLA’) with the FDA for TEVIMBRA as a treatment for patients with unresectable recurrent locally advanced or metastatic ESCC after prior systemic therapy. Additionally, the FDA accepted for review a BLA for TEVIMBRA as a first-line treatment for patients with unresectable, recurrent, locally advanced, or metastatic ESCC.
The company is evaluating tislelizumab in a broad pivotal clinical program for both solid tumor and hematological indications globally. BeiGene has launched more than 17 potentially registration-enabling trials with tislelizumab, of which 11 Phase 3 randomized trials and 4 Phase 2 trials have already had positive readouts. More than 900,000 patients have been prescribed TEVIMBRA globally to-date.
In September 2023, the company had entered into an agreement with Novartis to regain worldwide rights to develop, manufacture, and commercialize TEVIMBRA.
PARTRUVIX (pamiparib)
PARTRUVIX is a selective small molecule inhibitor of poly ADP-ribose polymerase 1 (‘PARP1’) and PARP2 enzymes. Pamiparib has demonstrated pharmacological properties such as brain penetration and PARP-DNA complex trapping in preclinical models.
In China, PARTRUVIX received conditional approval for the treatment of patients with germline BRCA (‘gBRCA’) mutation-associated recurrent advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with two or more lines of chemotherapy in May 2021. Full approval for this indication is contingent upon results from ongoing corroborative trials confirming the clinical benefit of PARTRUVIX in this population. PARTRUVIX was included in the 2021 NRDL in its approved indication.
In-Licensed Products from Amgen
The company is commercializing the following cancer medicines in China under an exclusive license from Amgen:
XGEVA
XGEVA (denosumab) is an antibody-based RANK ligand (‘RANKL’) inhibitor that was approved globally for the prevention of skeletal-related events (‘SREs’) in patients with bone metastases from solid tumors and in patients with multiple myeloma (‘MM’), and for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone (‘GCTB’). XGEVA is approved in over 70 countries worldwide. In China, XGEVA received conditional approval in the GCTB indication in May 2019 (converted to regular approval) and received conditional approval for the SRE indications in November 2020. The company began marketing XGEVA in China in July 2020. In December 2020, the company announced the inclusion of XGEVA in the NRDL for the treatment of GCTB, which was successfully renewed for inclusion in 2023. Beginning in January 2024, the SRE indication was also included in the NRDL.
BLINCYTO
BLINCYTO (blinatumomab), a bispecific CD-19 directed CD3 T-cell engager, is the first and only approved bi-specific T-cell engager (‘BiTE’) immunotherapy. It has been approved in 60 countries for use in patients with acute lymphoblastic leukemia (‘ALL’). In China, BLINCYTO received conditional approval as a treatment for adult patients with R/R ALL in December 2020 (converted to regular approval) and was conditionally approved in April 2022 for pediatric patients with R/R B-cell precursor ALL. The company began commercializing BLINCYTO in August 2021.
KYPROLIS
KYPROLIS (carfilzomib), a proteasome inhibitor, has been approved in over 60 countries for use in patients with R/R MM. It was approved in China as a treatment for patients with R/R MM in July 2021 and the company began commercializing KYPROLIS in January 2022. KYPROLIS was included on the NRDL beginning in March 2023 for its approved indication in China.
In-Licensed Products from BMS
The company commercializes the following cancer medicines in China under an exclusive license from BMS through 2024:
REVLIMID
REVLIMID (lenalidomide) is an oral immunomodulatory medicine that was approved in China in 2013 for the treatment of MM in combination with dexamethasone in adult patients who have received at least one prior therapy. In February 2018, REVLIMID received NMPA approval of a new indication for the treatment of MM in combination with dexamethasone in adult patients with previously untreated MM who are not eligible for transplant.
REVLIMID was listed on the NRDL in June 2017. In November 2019, the company announced that REVLIMID received formal inclusion on the NRDL in China for R/R MM. In November 2020 the company’s sNDA for the use of REVLIMID in combination with rituximab in adult patients with previously treated follicular lymphoma was approved by the NMPA.
VIDAZA
VIDAZA (azacitidine for injection) is a pyrimidine nucleoside analog that has been shown to reverse the effects of DNA hypermethylation and promote subsequent gene re-expression. VIDAZA was approved in China in April 2017 for the treatment of intermediate-2 and high-risk myelodysplastic syndromes (‘MDS’), chronic myelomonocyte leukemia (‘CMML’) and acute myeloid leukemia (‘AML’) with 20% to 30% blasts and multi-lineage dysplasia. In January 2018, VIDAZA became commercially available in China.
In addition to REVLIMID and VIDAZA, the company previously commercialized ABRAXANE (paclitaxel albumin-bound particles for injectable suspension), a solvent-free chemotherapy approved for use in certain patients with metastatic breast cancer, in China until March 2020. On March 25, 2020, the NMPA suspended the importation, sales and use of ABRAXANE in China previously supplied to the company by BMS, and the drug was subsequently recalled by BMS. This suspension was based on inspection findings at BMS’s contract manufacturing facility in the U.S. Following the suspension and recall of ABRAXANE, the company initiated an arbitration proceeding against BMS which has since been settled. As part of the settlement agreement, the license and supply agreement covering REVLIMID and VIDAZA was terminated as of December 31, 2023, subject to the company’s right to continuing selling inventory until it is sold out or December 31, 2024.
In-Licensed Products from EUSA Pharma
The company commercializes the following medicines in China under an exclusive license from EUSA Pharma:
SYLVANT
SYLVANT (siltuximab), an interleukin-6 (‘IL-6’) antagonist, was approved as a treatment for patients with idiopathic multicentric Castleman disease (‘iMCD’) who are human immunodeficiency virus (‘HIV’) negative and human herpesvirus-8 (‘HHV-8’) negative. SYLVANT was approved in China in December 2021 for the treatment of adult patients with multicentric Castleman disease (‘MCD’) who are HIV negative and HHV-8 negative, also known as iMCD. Beginning in January 2024, Sylvant was included in the NRDL.
QARZIBA
QARZIBA (dinutuximab beta), a mouse-human chimeric monoclonal GD2 antibody, was granted conditional approval by the NMPA for the treatment of high-risk neuroblastoma in patients aged 12 months and above who have previously received induction chemotherapy and achieved at least a partial response, followed by myeloablative therapy and stem cell transplantation, as well as patients with a history of R/R neuroblastoma with or without residual disease. The company began commercializing QARZIBA in December 2021.
In-Licensed Product from Bio-Thera
The company commercializes the following product in China under an exclusive license from Bio-Thera:
POBEVCY (BAT1706)
POBEVCY is a biosimilar to Avastin (bevacizumab) developed by Bio-Thera Solutions, Ltd., a commercial-stage biopharmaceutical company located in Guangzhou, China. In China, Avastin is approved for the treatment of patients with metastatic colorectal cancer, NSCLC, glioblastoma, ovarian, fallopian tube or primary peritoneal, and cervical cancers.
POBEVCY was approved by the NMPA in China in November 2021 and launched in late 2021 for the treatment of patients with advanced, metastatic or recurrent NSCLC, and metastatic colorectal cancer.
The company has acquired the right to develop, manufacture and commercialize POBEVCY in China, including Hong Kong, Macau, and Taiwan.
In-Licensed Product from Luye Pharma
The company commercializes the following product in China under an exclusive license from Luye Pharma:
BAITUOWEI (goserelin microspheres for injection)
Baituowei (Goserelin Microspheres for Injection), developed by Luye Pharma, is the world’s first and only approved microsphere formulation of Goserelin. With its innovative microsphere formulation, Baituowei is able to ensure efficacy and safety while significantly improving patient experience. Baituowei was approved by the NMPA in China in June 2023 for the treatment of patients with prostate cancer requiring androgen deprivation therapy and included in the NRDL in 2023, and was approved by the NMPA in China in September 2023 for treating breast cancer in premenopausal and perimenopausal women that can be treated with hormones.
Market Access
The company’s sales are largely dependent on the availability and extent of coverage and reimbursement by third party payors. In many markets these third parties are government health systems and in some markets, such as the U.S. there are also private payors such as private health insurers and health systems. As of December 31, 2023, the company had commercialized its products in over 65 markets.
Tislelizumab in eleven of its eligible approved indications:
In combination with fluoropyrimidine- and platinum-based chemotherapy, for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma with high PD-L1 expression (approved in February 2023 and included in the NRDL at the end of 2023);
In combination with paclitaxel plus platinum-based or fluoropyrimidine- plus platinum-based chemotherapy, for the first-line treatment of patients with unresectable locally advanced, recurrent or metastatic ESCC (approved in May 2023 and included in the NRDL at the end of 2023);
For the treatment of adult patients with locally advanced or metastatic non-squamous NSCLC who are negative for epidermal growth factor receptor (‘EGFR’) and anaplastic mesenchymal lymphoma kinase (‘ALK’) mutations and have progressed after or are intolerant of prior chemotherapy with platinum-containing regimens; and adult patients with locally advanced or metastatic squamous NSCLC who are negative or unknown for EGFR and ALK mutations and have progressed after or are intolerant of prior chemotherapy with platinum-containing regimens (approved in January 2022 and included in the NRDL at the beginning of 2023);
For the treatment of adult patients with advanced unresectable or metastatic MSI-H or dMMR solid tumors: patients with advanced colorectal cancer with disease progression after prior treatment with fluoropyrimidines, oxaliplatin and irinotecan; patients with other advanced solid tumors with disease progression after prior treatment and no satisfactory alternative treatment options (approved in March 2022 and included in the NRDL at the beginning of 2023);
For the treatment of patients with locally advanced or metastatic ESCC who have progressed after or are intolerant of prior first-line standard chemotherapy (approved in April 2022 and included in the NRDL at the beginning of 2023);
As a first-line treatment for patients with recurrent or metastatic NPC (approved in June 2022 and included in the NRDL at the beginning of 2023);
For use in combination with pemetrexed and platinum chemotherapy as a first-line treatment in patients with unresectable, locally advanced or metastatic non-squamous NSCLC, with EGFR genomic tumor aberrations negative and ALK genomic tumor negative (approved in June 2021 and included in the NRDL in 2021);
For the treatment of patients with HCC who have been previously treated with at least one systemic therapy (conditionally approved in June 2021 and included in the NRDL in 2021);
For use in combination with paclitaxel and carboplatin as a first-line treatment in patients with unresectable, locally advanced or metastatic squamous NSCLC (approved in January 2021 and included in the NRDL in 2021);
For the treatment of patients with locally advanced or metastatic UC with PD-L1 high expression whose disease progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (conditionally approved in April 2020 and included in the NRDL in 2020); and
For the treatment of patients with cHL who have received at least two prior therapies (conditionally approved in December 2019 and included in the NRDL in 2020).
BRUKINSA in all five of its approved indications:
For treatment-naïve patients with CLL or SLL (approved in April 2023 and included in the NRDL at the end of 2023);
For treatment-naïve patients with WM (approved in April 2023 and included in the NRDL at the end of 2023);
For the treatment of adult patients with WM who have received at least one prior therapy (conditionally approved in June 2021 and included in the NRDL in 2021; converted to regular approval in May 2023);
For the treatment of adult patients with MCL who have received at least one prior therapy (conditionally approved in June 2020 and included in the NRDL in 2020); and
For the treatment of adult patients with CLL/SLL who have received at least one prior therapy (conditionally approved in June 2020 and included in the NRDL in 2020; converted to regular approval in May 2023).
BAITUOWEI in one of its eligible approved indication:
For the treatment of patients with prostate cancer requiring androgen deprivation therapy (approved in June 2023 and included in the NRDL at the end of 2023).
SYLVANT in its approved indication:
For the treatment of adult patients with MCD who are HIV negative and HHV-8 negative (approved in December 2021 and included in the NRDL at the end of 2023).
XGEVA now listed as a Category B medicine and included all of the approved indications:
Prevention of skeletal-related events (fracture, spinal cord compression, or the need for radiation or surgery to bone) in patients with MM and in patients with bone metastases from solid tumors (approved in November 2020 and included in the NRDL at the end of 2023);
For the treatment of patients with GCTB that is unresectable or where surgical resection is likely to result in severe morbidity (first included in the NRDL in 2020).
PARTRUVIX successfully renewed its approved indication:
For the treatment of patients with gBRCA mutation-associated recurrent advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with two or more lines of chemotherapy (conditionally approved in May 2021 and included in the NRDL in 2021).
KYPROLIS in its approved indication:
In combination with dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma who have received at least two lines of therapy, including proteasome inhibitor and immunomodulator (approved in July 2021 and included in the NRDL at the beginning of 2023).
Additionally, two of the company’s medicines were listed in past NRDLs: REVLIMID was included in the 2017 NRDL negotiation list and later received formal inclusion in the 2019 Category B list, while VIDAZA was listed in the 2018 NRDL negotiation list and later received formal inclusion to the 2020 Category B list.
The company offers patient assistance programs in the U.S. under the company’s myBeiGene program. This program seeks to enhance access to BRUKINSA by assisting with obtaining reimbursement, co-pay assistance when allowed, temporary supply of free product for insurance delays, and free product assistance for some uninsured and underinsured patients. The programs also seek to support patients and caregivers by providing education and information about BRUKINSA and its approved indications, nurse advocates, and connecting patients to sources of support such as support groups and transportation/lodging assistance.
Commercial- and Clinical-Stage Drug Candidates
A description of the company’s commercial- and clinical-stage drug candidates and clinical data from selected clinical trials is set forth below. Historically, the company has made available, and the company intends to continue to make available, clinical data and/or topline results from clinical trials of the company’s drug candidates in its press releases and/or filings with the U.S. Securities and Exchange Commission (‘SEC’), the Stock Exchange of Hong Kong Limited (‘HKEx’), and the Shanghai Stock Exchange (‘SSE’), copies of which are available on the Investors section of the company’s website.
Commercial-Stage
BRUKINSA (zanubrutinib), a BTK Inhibitor
The company is evaluating zanubrutinib in a broad pivotal clinical program globally to treat a number of B-cell malignancies. Zanubrutinib has demonstrated sustained 24-hour BTK occupancy in both the peripheral blood and lymph node compartments in patients. Zanubrutinib is the only BTK inhibitor to demonstrate superior progression-free survival in R/R CLL versus IMBRUVICA (ibrutinib), an approved BTK inhibitor.
Overview of Clinical Development Program and Regulatory Status
The company has announced BRUKINSA approvals around the world, including in the U.S., China, the EU, the U.K., Canada, Australia, South Korea and Switzerland. As of December 2023, 32 additional marketing authorization applications for BRUKINSA have been submitted and are under review, including by BeiGene and with support from the company’s five distribution partners: Adium Pharma in Latin America and the Caribbean, NewBridge Pharmaceuticals in the Middle East and North Africa, Erkim in Turkey, Nanolek in Russia, and Medison in Israel.
Based on the clinical data as of December 31, 2023, BRUKINSA has a best-in-class profile, BeiGene has initiated broad global pivotal programs in multiple indications, including 11 registration or registration-enabling clinical trials. Six of the 11 trials are Phase 3, 3 are designed to be registration-enabling Phase 2 trials, and 2 are confirmatory trials.
ROSEWOOD A Global Randomized Study of Zanubrutinib plus Obinutuzumab vs Obinutuzumab Monotherapy in Relapsed/ Refractory Follicular Lymphoma (FL) (NCT03332017).
MAGNOLIA A Single-Arm Multicenter Study of Zanubrutinib subjects With Relapsed or Refractory Marginal Zone Lymphoma (MZL) (NCT03846427).
A Phase 2, Single-Arm, Open-Label, Multicenter Study of the Bruton Tyrosine Kinase Inhibitor Zanubrutinib in subjects With CD79B Mutant Relapsed/Refractory Diffuse Large B-Cell Lymphoma (NCT05068440).
ASPEN A Global Randomized Study of Zanubrutinib vs Ibrutinib in Subjects With Waldenström’s Macroglobulinemia (WM) (NCT03053440).
SEQUOIA A Global Randomized Study of Zanubrutinib vs Bendamustine Plus Rituximab in subjects With Previously Untreated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma (CLL/SLL) (NCT03336333).
ALPINE A Global Randomized Study of Zanubrutinib vs Ibrutinib in subjects With Relapsed/Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma (CLL/SLL) (NCT03734016).
MANGROVE A Randomized Global Study Comparing Zanubrutinib Plus Rituximab vs. Bendamustine Plus Rituximab in subjects With Previously Untreated Mantle Cell Lymphoma Who Are Ineligible for Stem Cell Transplantation (MCL) (NCT04002297).
MAHOGANY Zanubrutinib Plus Obinutuzumab vs Lenalidomide plus Rituximab in Relapsed/ Refractory Follicular Lymphoma (FL) (NCT05100862).
A Phase 2, Multicenter, Single-arm Study of Zanubrutinib in Patients With Previously Treated B-Cell Lymphoma Intolerant of Prior Treatment With Ibrutinib and/or Acalabrutinib.
A Global Randomized Study of zanubrutinib vs tacrolimus in Primary Membranous Nephropathy (NCT05707377).
A Multicenter, Randomized, Double Blind, Placebo Controlled Study to Evaluate the Safety and Efficacy of Zanubrutinib in Patients With Active Proliferative Lupus Nephritis (NCT04643470).
In addition, Nature Medicine (December 9, 2023) published compelling combination data of an investigator-initiated multicenter Phase 2 trial (NCT04271956) investigating BRUKINSA plus the company’s anti-PD-1 TEVIMBRA (tislelizumab) in CLL patients with Richter’s transformation (‘RT’). Extended follow-up data from ALPINE trial was presented at Annual American Society for Hematology (‘ASH’) 2023. In December 2023, the company received a U.S. Label Update which includes PFS superiority in R/R CLL (HR 0.65, p=0.0024); sustained with extended follow-up. Based on positive readout from ROSEWOOD study, EC has granted approval for the treatment of R/R FL. Finally, the company is also investigating zanubrutinib in several combination studies in MCL, MZL and CLL/SLL, including a Phase 3 trial in combination with sonrotoclax in front-line CLL/SLL. The company continues to examine opportunities for zanubrutinib combos with both sonrotoclax and tislelizumab.
The company continues to pursue regulatory approvals for BRUKINSA globally. Besides the company’s recent label updates in CLL/SLL in the U.S. (December 2023) and FL in EU (November 2023) the company expects continued regulatory decisions for some of the company’s global submissions this year, including potential additional approvals in more than 10 markets.
TEVIMBRA (tislelizumab), an anti-PD-1 Antibody
Tislelizumab is a humanized monoclonal antibody against the immune checkpoint receptor PD-1 that is being evaluated in pivotal clinical trials globally and for which the company plans to commence additional pivotal trials as a monotherapy and in combination with standard of care to treat various solid and hematological cancers.
Overview of Clinical Development Program and Regulatory Status
BeiGene has launched more than 17 potentially registration-enabling clinical trials globally, including 13 Phase 3 randomized trials and 4 Phase 2 trials intended to support regulatory submissions globally.
The company’s trials in lung cancer include:
A global Phase 3 trial evaluating tislelizumab as a second- or third-line treatment compared to docetaxel in patients with locally advanced or metastatic NSCLC (NCT03358875);
Two Phase 3 trials in China evaluating tislelizumab plus chemotherapy versus chemotherapy in squamous and non-squamous NSCLC (NCT03594747 and NCT03663205, respectively);
A Phase 3 trial in China in 1L SCLC evaluating tislelizumab plus chemotherapy versus chemotherapy (NCT04005716); and
A Phase 3 trial in China of tislelizumab in combination with platinum-based doublet chemotherapy as neoadjuvant treatment for patients with NSCLC (NCT04379635).
The company’s trials in liver cancer include:
A global Phase 3 trial comparing tislelizumab with sorafenib as first-line treatment for patients with HCC (NCT03412773); and
A global single-arm pivotal Phase 2 trial in second- or third-line unresectable HCC (NCT03419897).
The company’s trials in gastric cancer include:
A global Phase 3 trial of tislelizumab combined with chemotherapy versus placebo combined with chemotherapy as first-line treatment for patients with gastric cancer (NCT03777657).
The company’s trials in lymphoma include:
A Phase 3 trial in China comparing tislelizumab to salvage chemotherapy in patients with relapsed or refractory classical Hodgkin Lymphoma (cHL; NCT04486391); and
A Phase 2 trial in China in patients with relapsed or refractory cHL (NCT03209973).
The company’s trials in urothelial carcinoma include:
A Phase 3 trial in China in patients with locally advanced or metastatic urothelial carcinoma (NCT03967977); and
Phase 2 trial in China in patients with locally advanced or metastatic urothelial bladder cancer (NCT04004221).
The company’s trials in ESCC include:
A global Phase 3 trial comparing tislelizumab with chemotherapy as second-line treatment for patients with advanced ESCC (NCT03430843);
A global Phase 3 trial of tislelizumab in combination with chemotherapy as first-line treatment for patients with ESCC (NCT03783442); and
A Phase 3 trial in China of tislelizumab versus placebo in combination with chemoradiotherapy in patients with localized ESCC (NCT03957590).
Finally, the company’s trials in solid tumors and nasopharyngeal cancer include:
A Phase 2 trial in China in patients with MSI-H/dMMR solid tumors (NCT03736889); and
A Phase 3 trial in China and Thailand of tislelizumab combined with chemotherapy versus placebo combined with chemotherapy as first-line treatment in patients with nasopharyngeal cancer (NCT03924986).
As of December 2023, the company had enrolled over 13,000 subjects in clinical trials of tislelizumab in more than 30 countries, including 3,500+ subjects outside of China. These studies include 11 multi-regional registrational trials that are designed for global regulatory approvals. Data from the company’s trials thus far suggested that tislelizumab was generally well-tolerated and exhibited anti-tumor activity in a variety of tumor types.
PARTRUVIX (pamiparib), a PARP1 and PARP2 Inhibitor
Pamiparib is a selective small molecule inhibitor of PARP1 and PARP2 enzymes that is being evaluated as a potential monotherapy and in combinations for the treatment of various solid tumors.
Overview of Clinical Development Program and Regulatory Status
In China, pamiparib received conditional approval in May 2021 for the treatment of patients with gBRCA mutation-associated recurrent advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with two or more lines of chemotherapy. Full approval for this indication is contingent upon results from ongoing corroborative trials confirming the clinical benefit of pamiparib in this population.
In addition, the company’s clinical development program includes a Phase 3 trial as a maintenance therapy in patients with platinum-sensitive recurrent OC (NCT03519230).
Clinical-Stage
Sonrotoclax (BGB-11417), a Small Molecule Bcl-2 Inhibitor
BGB-11417 is an investigational small molecule Bcl-2 inhibitor that is in late-stage clinical trials. The company has completed preclinical studies and has ongoing investigational new drug (‘IND’) -enabling studies of BGB-11417, which demonstrate potent activity and high selectivity against the pro-apoptotic protein Bcl-2. The molecule appears to be more potent than venetoclax and shows the potential to common venetoclax resistance mutations. Further, it is more selective than venetoclax for Bcl-2 relative to Bcl-xL. Designed with a shorter half-life, this will lead to a more favorable tolerability profile and better handling. Finally, it is well-positioned to be ideal backbone for combination therapies, especially with zanubrutinib and the company’s BTK-CDAC in fixed duration treatment strategy.
The preliminary clinical data on pharmacokinetic/pharmacodynamic (‘PK/PD’), efficacy and safety from more than 700 patients enrolled by December 2023 in different indications and in different combinations are consistent with the preclinical best-in-class hypothesis of a more potent and selective BCL2 inhibitor compared to venetoclax. In TN-CLL sonrotoclax was safe and tolerable in combination with BRUKINSA; with deep and durable responses.
Clinical data of a Phase 1 trial investigating TN-CLL/SLL, MZL (NCT04277637) and 1/ 2 trials investigating MM with t(11,14) was presented at ASH 2023. With these encouraging results, the company’s BCL2 inhibitor sonrotoclax (BGB-11417) program is steadily progressing at pivotal stage. Clinical trials with registration-potential have already been initiated in R/R MCL globally (NCT05471843), R/R WM globally (NCT05952037) and R/R CLL/SLL for China only (NCT05479994). Notably, the company has just initiated the Phase 3 study of sonrotoclax + BRUKINSA vs venetoclax + obinutuzumab to support the development of sonrotoclax in the 1L CLL indication. This study has started enrollment.
BGB-16673, a BTK-targeted CDAC
BGB-16673 is an investigational BTK-targeting chimeric degradation activation compound (‘CDAC’) active against both wild-type and mutant BTK. BGB-16673 has demonstrated preclinical antitumor activity in models with wild-type BTK and models with BTK inhibitorresistant mutations commonly observed in patients who have progressed on prior BTK inhibitor treatment. A Phase 1 open-label, dose-escalation, and dose-expansion trial (NCT05006716) in adult patients with R/R B-cell malignancies is enrolling. Preliminary results are presented at ASH 2023. The company expects to initiate dose expansion in 2024.
BGB-10188, a PI3Kd Inhibitor
BGB-10188 is an investigational PI3Kd inhibitor being evaluated in a Phase 1 clinical trial (NCT04282018) as monotherapy or in combination with BRUKINSA in hematology malignancies, or in combination with tislelizumab in solid tumors.
BGB-21447, a Bcl-2 Inhibitor
BGB-21447 is an investigational Bcl-2 inhibitor being evaluated in a Phase 1 clinical trial (NCT05828589) as monotherapy in mature B-cell malignancies. In preclinical studies, BGB-21447 shows additional potency and selectivity, with a longer half-life than sonrotoclax. The differentiated profiles of sonrotoclax and BGB-21447 may lead to unique development options for these programs.
Ociperlimab (BGB-A1217), a TIGIT Inhibitor
Ociperlimab (BGB-A1217) is an investigational humanized IgG1-variant monoclonal antibody directed against TIGIT. An immune checkpoint molecule, ociperlimab is being investigated in a global Phase 3 trial, AdvanTIG-302 (NCT04746924), in combination with tislelizumab in NSCLC. As of December 2023, more than 2,000 subjects have been enrolled across the ociperlimab development program, which includes eight global trials in patients with lung cancers, esophageal squamous cell carcinoma, and cervical cancer.
In December 2021 the company announced an option, collaboration and license agreement with Novartis to develop, manufacture and commercialize ociperlimab in North America, Europe, and Japan. In July 2023, the agreement was mutually terminated and the company regained the full global rights for ociperlimab.
Due to the changing treatment paradigm, the company discontinued AdvanTIG 301 (NCT04866017), a Phase 3 trial of ociperlimab in combination with its anti-PD-1 antibody tislelizumab versus AstraZeneca’s Imfinzi (durvalumab) following concurrent chemoradiotherapy in patients with stage III unresectable NSCLC.
The company will continue patient enrollment in AdvanTIG-302, while evaluating all available data to inform future development opportunities with ociperlimab.
Zanidatamab, a bispecific HER2-targeted antibody
Zanidatamab, a novel investigational Azymetric bispecific antibody targeting HER2, is in late-stage clinical development with Zymeworks Inc. BeiGene has development and commercial rights to zanidatamab in Asia (excluding Japan), Australia, and New Zealand. The company is participating in three ongoing clinical studies with zanidatamab. The first is a Phase 1/2 study (NCT04215978) in HER2-positive breast and gastric cancer. The breast cancer arm combines zanidatamab with docetaxel, and the gastric cancer arm combines zanidatamab with the company’s PD-1 inhibitor tislelizumab and chemotherapy. The second study HERIZON-BTC-01 (NCT04466891) is a Phase 2b study in patients with advanced or metastatic HER2-amplified biliary tract cancers (‘BTC’) in which zanidatamab is being used as monotherapy. Positive topline results from this trial were announced in 2022 and presented at ASCO 2023. The company is continuing the global Phase 3 clinical trial (NCT05152147) examining zanidatamab in combination with chemotherapy with and without tislelizumab in HER2-positive gastroesophageal cancer.
Surzebiclimab (BGB-A425), a TIM-3 Inhibitor
Surzebiclimab (BGB-A425) is an investigational humanized IgG1-variant monoclonal antibody against T-cell immunoglobulin and mucin-domain containing-3. The company has an ongoing Phase 2 trial (NCT03744468) of surzebiclimab in combination with tislelizumab in NSCLC and HNSCC.
BGB-A445, an OX40 Agonist Antibody
BGB-A445 is an investigational agonistic antibody directed to the OX40 antigen. BGB-A445 is a non-ligand competing antibody that does not disrupt OX40 to OX40 ligand engagement. Preclinical experiments showed that BGB-A445 has increasing effectiveness at higher doses versus an antibody that was ligand-competing, which showed falling effectiveness at higher doses. BGB-A445 has also showed in preclinical tests the potential to be combined with several agents. BGB-A445 is being evaluated in a Phase 1 trial (NCT04215978) in patients with advanced solid tumors, a Phase 2 basket trial in melanoma, renal cell cancer and bladder cancer (NCT05661955), a Phase 2 umbrella trial in 1L NSCLC (NCT05635708), and a Phase 2 umbrella trial in 2L+ NSCLC (NCT06029127).
BGB-15025, a Small Molecule HPK1 Inhibitor
BGB-15025 is an investigational small molecule inhibitor of HPK1, which is a key negative feedback regulator of TCR signaling. Inhibition of HPK1 leads to enhanced T-cell activation pre-clinically. In addition, preclinical studies showed that BGB-15025 exhibits combination activity with tislelizumab and has a wide therapeutic window. The company initiated a Phase 1 trial (NCT04649385) of BGB-15025 alone and in combination with tislelizumab in patients with advanced solid tumors in 2021. This trial is being conducted in multiple countries globally and has entered dose expansion. BGB-15025 is also being tested in a 1L NSCLC umbrella study in combination with tislelizumab and other novel agents.
BGB-24714, a SMAC mimetic
BGB-24714 is an investigational Second Mitochondrial-derived Activator of Caspase (‘SMAC’) mimetic enrolling in a Phase 1 clinical trial (NCT05381909) as monotherapy and in combination with paclitaxel, or in combination with chemoradiotherapy in advanced solid tumors.
BGB-26808, a HPK-1 Inhibitor
BGB-26808 is a second-generation HPK-1 inhibitor with a different scaffold from BGB-15025 being evaluated in a Phase 1 clinical trial (NCT05981703) as monotherapy or in combination with tislelizumab in participants with advanced solid tumors.
Lifirafenib (BGB-283) and BGB-3245, Inhibitors of RAF
Lifirafenib is an investigational novel small molecule inhibitor with RAF monomer and dimer inhibition activities. Lifirafenib has shown antitumor activities in preclinical models and in cancer patients with tumors harboring BRAF V600E mutations, non-V600E BRAF mutations or KRAS/NRAS mutations. The company has been developing lifirafenib for the treatment of cancers with aberrations in the mitogen-activated protein kinase (‘MAPK’), pathway, including BRAF gene mutations and KRAS/NRAS gene mutations where first generation BRAF inhibitors are not effective. Lifirafenib as monotherapy or in combination with other agents may have potential for treating various malignancies such as melanoma, NSCLC, and endometrial cancer.
BeiGene is working together with SpringWorks Therapeutics, Inc. (‘SpringWorks’) in a global clinical collaboration and has initiated a Phase 1b clinical trial (NCT03905148) to evaluate the safety, tolerability, and preliminary efficacy of lifirafenib in combination with SpringWorks’ investigational MEK inhibitor, mirdametinib (PD-0325901), in patients with advanced solid tumors.
In addition to the collaboration, BeiGene and SpringWorks formed a separate company, MapKure, LLC, to develop BGB-3245, an investigational, selective next-generation RAF kinase inhibitor discovered by BeiGene scientists. MapKure has an ongoing Phase 1 clinical trial of BGB-3245 (NCT04249843) in patients with advanced or refractory tumors harboring specific v-RAF murine sarcoma viral oncogene homolog B (‘B-RAF’) genetic mutations.
BGB-30813, a DGKZeta Inhibitor
BGB-30813 is an investigational DGKzeta inhibitor being evaluated in a Phase 1 clinical trial (NCT05904496) as monotherapy and in combination with tislelizumab in participants with advanced or metastatic solid tumors.
BGB-A3055, an anti-CCR8 Antibody
BGB-A3055 is an investigational anti-CCR8 antibody being evaluated in a Phase 1 clinical trial (NCT05935098) as monotherapy or in combination with tislelizumab in participants with advanced or metastatic solid tumors.
BGB-43395, a CDK-4 Inhibitor
BGB-43395 is an investigational CDK4 inhibitor being evaluated in a Phase 1 clinical trial (NCT06120283) as monotherapy or in combination with either fulvestrant or letrozole in participants with hormone receptor positive (‘HR+’) and human epidermal growth factor 2 negative (‘HER2-’) breast cancer (‘BC’) and other advanced solid tumors.
Preclinical Programs
The company has a proprietary biology research platform that has allowed the company to research and develop both small molecules and biologic molecules. In the last decade, this platform has generated more than 10 clinical stage assets, including three internally-developed molecules that have been approved by regulatory bodies in the U.S., China, EU, and other markets, with other filings pending globally and planned to be submitted. The platform is a full-process technology system spanning from early discovery to commercialization of oncology medicines based on multiple drug technology platforms that can be applied to oncology and other fields. The company has core technology platforms for the development of small molecule and antibody medicines and the manufacturing of the company’s own and potentially other medicines. The company has over 50 preclinical programs and the majority have best-in-class or first-in-class potential.
The company anticipates advancing many of its preclinical drug candidates into the clinic in the next 12 months. The company has the opportunity to combine tislelizumab with the company’s preclinical candidates to target multiple points in the cancer immunity cycle. The company also may seek to develop companion diagnostics that will help identify patients who are most likely to benefit from the use of the company’s medicines and drug candidates.
Manufacturing and Supply
The company entered into a commercial supply agreement with Catalent Pharma Solutions, LLC (‘Catalent’) to produce BRUKINSA at Catalent’s Kansas City, Missouri site and have added Catalent’s facility in Winchester, Kentucky for clinical and commercial use in the U.S. and other countries outside of China. The company sources the active pharmaceutical ingredient for BRUKINSA from a supplier in China and full qualification of a supplier outside of China is near completion and will be soon active. In addition, the company entered into a commercial supply agreement with Boehringer Ingelheim Biopharmaceuticals (China) Ltd. (‘Boehringer Ingelheim’) for tislelizumab, which is being manufactured at Boehringer Ingelheim’s facility in Shanghai, China. Additionally, the company entered into an agreement with Novartis to regain worldwide rights to develop, manufacture, and commercialize tislelizumab. The company will continue to work with Novartis on development, regulatory and manufacturing priorities. Novartis will manufacture tislelizumab for many markets worldwide and explore its potential in combination with their oncology assets. For the company’s commercial and clinical stage products in-licensed from Amgen, BMS and others, the company rely on the licensors and their manufacturing facilities or their CMOs for the supply of those medicines and drug candidates.
The core raw materials used in manufacturing at the company’s Guangzhou facility are genetically modified cell lines that the company has co-developed and licensed from Boehringer Ingelheim and other third parties.
Amgen Collaboration
Collaboration Agreement
On October 31, 2019, the company’s wholly-owned subsidiary, BeiGene Switzerland GmbH (‘BeiGene Switzerland’), entered into a Collaboration Agreement with Amgen, which became effective on January 2, 2020 (as amended, the ‘Amgen Collaboration Agreement’). Pursuant to the terms of the Amgen Collaboration Agreement, the company is responsible for commercializing Amgen’s oncology products XGEVA, BLINCYTO and KYPROLIS in China (excluding Hong Kong, Macao and Taiwan) for a period of five or seven years following each product’s regulatory approval in China, as specified in the Amgen Collaboration Agreement, with the commercialization period for XGEVA commencing following the transition of operational responsibilities for the product. In addition, as specified in the agreement, the company has the option to retain one of the three products to commercialize for as long as the product is sold in China. The parties have agreed to equally share profits and losses for the products in China during each product’s commercialization period. After expiration of the commercialization period for each product, the products not retained will be transitioned back to Amgen and the company will be eligible to receive tiered mid-single to low-double digit royalties on net sales in China of each product for an additional five years.
Additionally, pursuant to the terms of the Amgen Collaboration Agreement, the company and Amgen have agreed to collaborate on the global clinical development and commercialization of a portfolio of Amgen clinical- and late-preclinical-stage oncology pipeline products.
In connection with the company’s ongoing assessment of the Amgen Collaboration Agreement cost-share contributions, the company determined that the company’s further investment in the development of AMG 510 was no longer commercially viable for BeiGene. As a result, in February 2023, the company entered into an amendment to the Amgen Collaboration Agreement to (i) stop sharing costs with Amgen for the further development of AMG 510 during the period starting January 1, 2023 and ending August 31, 2023; and (ii) cooperate in good faith to prepare a transition plan with the termination of AMG 510 from the Amgen Collaboration Agreement.
Intellectual Property
As of February 14, 2024, the company owned 63 issued U.S. patents, 46 issued China patents, a number of pending U.S. and China patent applications, and corresponding patents and patent applications internationally.
Under the company’s collaboration with Amgen, the company has the right to commercialize three medicines in China. The company has one in-licensed medicine in China from Shandong Luye Pharmaceutical Co., Ltd (‘Luye’).
Additionally, the company owns a number of registered trademarks and pending trademark applications. The company has registered trademarks for BeiGene, the company’s corporate logo and product names and logos in the U.S., China, the EU and other jurisdictions, and the company is seeking further trademark protection for BeiGene, the company’s corporate logo, product names and logos, and other marks in jurisdictions where available and appropriate.
Government Regulation
Any products for which the company receives FDA approval are subject to continuing regulation by the FDA, including among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements.
Research and Development
The company’s research and development expense included $1.8 billion for the year ended December 31, 2023.
History
BeiGene, Ltd. was founded in 2010. The company was incorporated in the Cayman Islands in 2010.
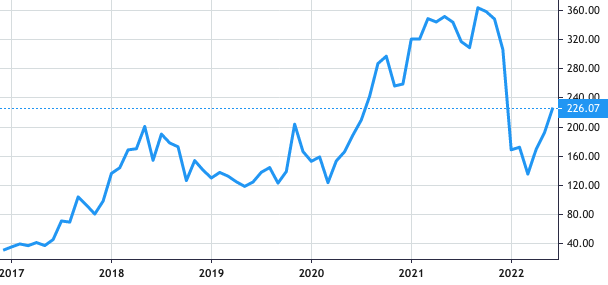
 Stock Value
Stock Value

