About Arrowhead Pharmaceuticals
Arrowhead Pharmaceuticals, Inc. develops medicines that treat intractable diseases by silencing the genes that cause them.
Using a broad portfolio of RNA chemistries and efficient modes of delivery, the company's therapies trigger the RNA interference mechanism to induce rapid, deep and durable knockdown of target genes.
The company's pipeline of 14 clinical stage investigational medicines range in development stage from Phase 1 to Phase 3. In addition, the company has a robust discovery stage pipeline, which is capable of generating multiple new clinical candidates each year.
Targeted RNAi Molecule (TRiM) Platform
The company's Targeted RNAi Molecule (TRiM) platform utilizes ligand-mediated delivery and is designed to enable tissue-specific targeting while being structurally simple. Targeting has been core to the company's development philosophy and the TRiM platform builds on more than a decade of work on actively targeted drug delivery vehicles. The company's scientists have discovered ways to progressively 'TRiM' away extraneous features and chemistries and retain optimal pharmacologic activity.
The TRiM platform comprises a highly potent RNA trigger identified using the company's proprietary trigger selection rules and algorithms with the following components optimized, as needed, for each drug candidate: a high affinity targeting ligand; various linker and chemistries; structures that enhance pharmacokinetics; and highly potent RNAi triggers with sequence specific stabilization chemistries.

The company is leading the expansion with the TRiM platform, which has shown the potential to reach multiple tissues, including liver, lung, central nervous system (CNS), muscle, and adipose tissue.
RNA Chemistries
The company's broad portfolio of RNA trigger structures and chemistries, including some proprietary structures, enable it to optimize each drug candidate on a target-by-target basis and utilize the combination of structure and chemical modifications that yield the most potent RNA interference (RNAi) trigger.
As a component of the TRiM platform, the company's design philosophy for RNA chemical modifications is to start with a structurally simple molecule and add only selective modification and stabilization chemistries as necessary to achieve the desired level of target knockdown and duration of effect.
Pipeline
The company focuses on developing innovative drugs for diseases with a genetic basis, typically characterized by the overproduction of one or more proteins that are involved with disease. The depth and versatility of the company's RNAi technologies enables it to potentially address conditions in virtually any therapeutic area and pursue disease targets that are not otherwise addressable by small molecules and biologic. The company is focused on bringing the promise of RNAi to address diseases outside of the liver, and its pipeline now includes disease targets in the liver, lung, muscle, and CNS.
Plozasiran
Plozasiran is designed to reduce production of Apolipoprotein C-III (apoC-III), a component of triglyceride rich lipoproteins (TRLs), including Very Low Density Lipoprotein (VLDL) and chylomicrons and a key regulator of triglyceride metabolism. The company is investigating plozasiran in two Phase 2b clinical trials, one Phase 3 clinical trial, and additional Phase 3 clinical trials on schedule to begin in 2024.
Zodasiran
Zodasiran is designed to reduce production of angiopoietin-like protein 3 (ANGPTL3), a liver synthesized inhibitor of lipoprotein lipase and endothelial lipase. ANGPTL3 inhibition has been shown to lower serum LDL, serum and liver triglyceride and has genetic validation as a novel target for cardiovascular disease. The company is investigating zodasiran in two Phase 2b clinical trials.
ARO-PNPLA3
ARO-PNPLA3 is an investigational RNAi therapeutic designed to reduce liver expression of patatin-like phospholipase domain containing 3 (PNPLA3) as a potential treatment for patients with non-alcoholic steatohepatitis (NASH). PNPLA3 has strong genetic and preclinical validation as a driver of fat accumulation and damage in the livers of patients who carry the common I148M mutation. Former licensee Janssen Pharmaceuticals, Inc. investigated ARO-PNPLA3 in two Phase 1 clinical trials and the company is designing a Phase 2 clinical trial.
ARO-RAGE
ARO-RAGE is designed to reduce production of the Receptor for Advanced Glycation End products (RAGE) as a potential treatment for various inflammatory pulmonary diseases. The company is investigating ARO-RAGE in a Phase 1/2a clinical trial.
ARO-MUC5AC
ARO-MUC5AC is designed to reduce production of mucin 5AC (MUC5AC) as a potential treatment for various muco-obstructive pulmonary diseases. The company is investigating ARO-MUC5AC in a phase 1/2a clinical trial.
ARO-MMP7
ARO-MMP7 is designed to reduce expression of matrix metalloproteinase 7 (MMP7) as a potential treatment for idiopathic Pulmonary Fibrosis (IPF). The company is investigating ARO-MMP7 in a Phase 1/2a clinical trial.
ARO-DUX4
ARO-DUX4 is designed to target the gene that encodes human double homeobox 4 (DUX4) protein as a potential treatment for patients with facioscapulohumeral muscular dystrophy.
ARO-SOD1
ARO-SOD1 is designed to target the gene that encodes human superoxide dismutase 1 (SOD1) protein as a potential treatment for patients with amyotrophic lateral sclerosis (ALS) harboring a SOD1 mutations.
ARO-C3
ARO-C3 is designed to reduce production of complement component 3 (C3) as a potential therapy for patients with various complement mediated or complement associated renal diseases. The company is investigating ARO-C3 in a Phase 1/2a clinical trial.
Collaboration and License Agreements
Glaxosmithkline Intellectual Property (No. 3) Limited (GSK)
On November 22, 2021, GSK and the company entered into an Exclusive License Agreement (the GSK License Agreement). Under the GSK License Agreement, GSK has received an exclusive license for GSK-4532990. The exclusive license is worldwide with the exception of greater China. GSK is wholly responsible for all clinical development and commercialization of GSK-4532990 in its territory.
GSK-4532990
GSK-4532990 is designed to reduce production of HSD17B13, a hydroxysteroid dehydrogenase involved in the metabolism of hormones, fatty acids and bile acids. GSK is conducting a Phase 2b clinical trial.
Horizon Therapeutics Ireland DAC (Horizon)
On June 18, 2021, Horizon and the company entered into a collaboration and license agreement (the Horizon License Agreement). Under the terms of the Horizon License Agreement, Horizon received a worldwide exclusive license for HZN-457, a clinical-stage medicine being developed by Horizon as a potential treatment for people with uncontrolled gout. Horizon is wholly responsible for clinical development and commercialization of HZN-457. On October 6, 2023, Amgen completed its acquisition of Horizon.
HZN-457
HZN-457 is designed to reduce production of xanthine dehydrogenase (XDH) as a potential treatment for people with uncontrolled gout.
Takeda Pharmaceutical Company Limited (Takeda)
On October 7, 2020, Takeda and the company entered into an Exclusive License and Co-Funding Agreement (the Takeda License Agreement). Under the Takeda License Agreement, Takeda and the company co-develops its Fazirsiran program the company's second-generation subcutaneously administered RNAi therapeutic candidate being developed as a treatment for liver disease associated with alpha-1 antitrypsin deficiency. Within the United States, fazirsiran, if approved, will be co-commercialized under a 50/50 profit sharing structure. Outside the United States, Takeda received an exclusive license to commercialize fazirsiran and will lead the global commercialization strategy.
Fazirsiran
Fazirsiran is a subcutaneously administered RNAi therapeutic being developed as a treatment for liver disease associated with alpha-1 antitrypsin deficiency (AATD), which is a rare genetic disorder that severely damages the liver and lungs of affected individuals. Fazirsiran is designed to reduce production of the mutant Z-AAT protein by silencing the AAT gene in order to prevent accumulation of Z-AAT in the liver, allow clearance of the accumulated Z-AAT protein, prevent repeated cycles of cellular damage, and possibly prevent or even reverse the progression of liver fibrosis.
Amgen Inc. (Amgen)
On September 28, 2016, Amgen and the company entered into two collaboration and license agreements and a common stock purchase agreement. Under the Second Collaboration and License Agreement (the Olpasiran Agreement), Amgen received a worldwide, exclusive license to the company's novel RNAi olpasiran program. These RNAi molecules are designed to reduce elevated lipoprotein(a), which is a genetically validated, independent risk factor for atherosclerotic cardiovascular disease. Under the Olpasiran Agreement, Amgen is wholly responsible for clinical development and commercialization.
Olpasiran
Olpasiran is designed to reduce the production of apolipoprotein A, a key component of lipoprotein(a), which has been genetically linked with increased risk of cardiovascular diseases, independent of cholesterol and LDL levels. Amgen completed a Phase 2 clinical study evaluating the efficacy, safety, and tolerability of olpasiran in subjects with elevated levels of lipoprotein(a). Amgen reported Phase 2 clinical results at the American Heart Association (AHA) Scientific Sessions in November 2022 and simultaneously published in the New England Journal of Medicine. Amgen began evaluating olpasiran in a Phase 3 study to assess the impact of olpasiran on major cardiovascular events in participants with atherosclerotic cardiovascular disease and elevated lipoprotein(a), in a double-blind, randomized, placebo-controlled, multi center study in December 2022.
Joint Venture and License Agreement with Visirna Therapeutics, Inc. (Visirna)
On April 25, 2022, Visirna and the company entered into a License Agreement (the Visirna License Agreement), pursuant to which Visirna received an exclusive license to develop, manufacture, and commercialize four of the company's RNAi-based investigational cardiometabolic medicines in Greater China (including the People's Republic of China, Hong Kong, Macau, and Taiwan). Pursuant to a Share Purchase Agreement entered into simultaneously with the Visirna License Agreement (the Visirna SPA), the company acquired a majority stake in Visirna (after accounting for shares reserved for Visirna's employee stock ownership plan) as partial consideration for the Visirna License Agreement.
Intellectual Property and Other Key Agreements
The company controls approximately 534 issued patents (including 342 directed to RNAi trigger molecules; 89 directed to targeting groups or targeting compounds; and one for hydrodynamic gene delivery), including European validations, and approximately 793 pending patent applications worldwide from 79 different patent families. The company's patent applications have been filed throughout the world, including, in the United States, Argentina, ARIPO (Africa Regional Intellectual Property Organization), Australia, Brazil, Canada, Chile, China, Eurasian Patent Organization, Europe, GCC (Gulf Cooperation Council), Hong Kong, Israel, India, Indonesia, Iraq, Jordan, Japan, Lebanon, Mexico, New Zealand, OAPI (African Intellectual Property Organization), Peru, the Philippines, Russia, South Africa, Saudi Arabia, Singapore, South Korea, Thailand, Taiwan, Uruguay, Venezuela, and Vietnam.
RNAi Triggers: The company owns issued patents or has filed patent applications directed to RNAi trigger molecules, which serve as the foundation of its TRiM platform, and are targeted to reduce expression of various gene targets. These patents and patent applications include the following:
Delivery Technologies: The delivery technology-related patents and patent applications, which include components used in the company's TRiM platform, have been filed and/or issued in various jurisdictions worldwide, including the United States, Argentina, Australia, Brazil, Canada, China, Eurasian Patent Organization, Europe (including validations in France, Germany, Italy, Spain, Switzerland, and the United Kingdom), GCC (Gulf Cooperation Council), Israel, India, Japan, Lebanon, Mexico, New Zealand, the Philippines, Russia, South Africa, South Korea, Singapore, Taiwan, and Uruguay. The company also controls a patent directed to hydrodynamic nucleic acid delivery that issued in the United States.
Non-Exclusively Licensed Patent Rights from Roche
On October 21, 2011, the company acquired the RNAi therapeutics business of Hoffmann-La Roche, Inc. and F. Hoffmann-La Roche Ltd. (collectively, Roche). Pursuant to this acquisition, Roche assigned to the company its entire rights under certain licenses, including the License and Collaboration Agreement between Roche and Alnylam dated July 8, 2007 (the Alnylam License); the Non-Exclusive Patent License Agreement between Roche and MDRNA, Inc. dated February 12, 2009 (MDRNA License); and the Non-Exclusive License Agreement between Roche and City of Hope dated September 19, 2011 (the COH License) (collectively the RNAi Licenses).
The RNAi Licenses include licenses to patents related to modifications of double-stranded oligonucleotides, including modifications to the base, sugar, or internucleoside linkage, nucleotide mimetics, and end modifications, which do not abolish the RNAi activity of the double-stranded oligonucleotides. Also included are patents relating to modified double-stranded oligonucleotides, such as meroduplexes described in U.S. Patent No. 9,074,205 assigned to Marina Biotech (f/k/a MDRNA, Inc.), as well as U.S. Patent Nos. 8,314,227; 9,051,570; and 9,303,260 related to unlocked nucleotide analogs (UNA). The UNA patents were assigned by Marina Biotech to Arcturus Therapeutics, Inc., but remain part of the MDRNA License. The RNAi Licenses further include patents related to dicer substrates and uses of the double-stranded oligonucleotides that function through the mechanism of RNA interference, such as described in City of Hope's U.S. Patent Nos. 8,084,599; 8,658,356; 8,691,786; 8,796,444; 8,809,515; and 9,518,262.
Government Regulation
The collection and use of personal health data and other personal data in the EU is governed by the provisions of the European General Data Protection Regulation (EU) 2016/679 ("GDPR"), which came into force in May 2018 and related implementing laws in individual EU member states. The GDPR increased responsibility and liability in relation to personal data that the company processes.
History
Arrowhead Pharmaceuticals, Inc. was founded in 2003.
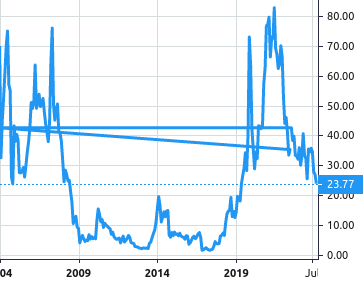
 Stock Value
Stock Value

