About Xenon Pharmaceuticals
Xenon Pharmaceuticals Inc. operates as a clinical stage biopharmaceutical company. The company is committed to delivering innovative therapeutics to improve the lives of patients with neurological disorders. The company is advancing a novel product pipeline of neurology-focused therapies to address areas of high unmet medical need, with a focus on epilepsy. In addition to its proprietary product candidates, the company has partnered programs with academic and industry collaborators, including Neurocrine Biosciences, Inc., or Neurocrine Biosciences.
Strategy
The key components of the company’s strategy include leveraging its discovery capabilities – which were founded upon its understanding of the genetics of channelopathies combined with proprietary biology and medicinal chemistry assets and know-how – to identify product candidates for development, drug targets and/or new indications for its existing product candidates; advancing selected proprietary product candidates through clinical development; selectively establishing collaborations that allow the company to potentially expand its internal capabilities and/or address broader commercial opportunities than may be possible independently; identifying opportunities to further expand its pipeline though indication expansion, acquisition, or in-licensing of external product candidates; and commercializing product candidates alone or in collaboration with others.
Pipeline
Product Candidates
Overview of XEN1101, A Kv7 Potassium Channel Opener

XEN1101 is a differentiated Kv7 potassium channel opener being developed for the treatment of epilepsy and other neurological disorders, including major depressive disorder, or MDD. The Kv7 potassium channel mechanism has been clinically validated with ezogabine, an earlier generation Kv7 opener that was approved by the U.S. Food and Drug Administration, or FDA, as an adjunctive treatment for adults with focal seizures with or without secondary generalization. XEN1101’s unique composition is chemically designed to improve upon potency, selectivity and pharmacokinetics, or PK, of ezogabine.
XEN1101 for Focal Onset Seizures (FOS)
In October 2021, the company announced positive results from its Phase 2b X-TOLE clinical trial, which evaluated the clinical efficacy, safety and tolerability of XEN1101 administered as an adjunctive treatment for adult patients with focal epilepsy. The topline data showed all primary and secondary seizure reduction endpoints were statistically significant across all dose groups, including the primary endpoint of median percent change, or MPC, from baseline in monthly seizure frequency and in the key secondary endpoint of patients with at least a 50% reduction in monthly focal seizure frequency from baseline, with p-values of <0.001 for both the 20 mg and 25 mg dose groups.
Following the positive results of its Phase 2b X-TOLE clinical trial, the company had an End-of-Phase 2 meeting with the FDA and initiated its XEN1101 Phase 3 development program, which includes two identical Phase 3 clinical trials to be run in parallel, called X-TOLE2 and X-TOLE3, that are designed closely after the Phase 2b X-TOLE clinical trial. These multicenter, randomized, double-blind, placebo-controlled trials will evaluate the clinical efficacy, safety, and tolerability of XEN1101 administered as adjunctive treatment in approximately 360 patients per study with focal onset seizures, or FOS. The primary efficacy endpoint is the MPC in monthly seizure frequency from baseline through the double-blind period, or DBP, of XEN1101 compared to placebo.
XEN1101 for Primary Generalized Tonic Clonic Seizures (PGTCS)
The company has initiated a Phase 3 clinical trial, called X-ACKT, to support potential regulatory submissions in an additional epilepsy indication of primary generalized tonic clonic seizures, or PGTCS. This multicenter, randomized, double-blind, placebo-controlled study will evaluate the clinical efficacy, safety, and tolerability of XEN1101 administered as adjunctive treatment in approximately 160 patients with PGTCS. The primary efficacy endpoint is the MPC in monthly PGTCS frequency from baseline through the DBP of XEN1101 compared to placebo.
Upon completion of the DBP in X-TOLE2, X-TOLE3, or X-ACKT, eligible patients may enter an open-label extension, or OLE, study for up to three years. In addition, the ongoing X-TOLE Phase 2b OLE continues to generate important long-term data for XEN1101.
XEN1101 for Major Depressive Disorder (MDD)
Based on promising pre-clinical data with XEN1101 and published clinical data generated using ezogabine, the company is evaluating the clinical efficacy, safety and tolerability of XEN1101 administered as monotherapy in approximately 150 patients with MDD in a Phase 2 clinical trial called X-NOVA. Designed as a randomized, double-blind, placebo-controlled, multicenter clinical study, the primary objective is to assess the efficacy of XEN1101 compared to placebo on improvement of depressive symptoms in subjects diagnosed with moderate to severe MDD, using the Montgomery-Åsberg Depression Rating Scale, or MADRS, score change through week six. Topline results from the X-NOVA study are anticipated in the third quarter of 2023.
The company is also collaborating with the Icahn School of Medicine at Mount Sinai to support an ongoing investigator-sponsored Phase 2 proof-of-concept, randomized, parallel-arm, placebo-controlled multi-site study of XEN1101 for the treatment of MDD in approximately 60 subjects.
In 2022, the company presented additional sub-group analyses of data from the XEN1101 Phase 2b X-TOLE clinical trial and interim data from the X-TOLE OLE. Additional sub analyses of the X-TOLE data suggest that the rapid onset of efficacy for XEN1101 was associated with starting at an effective, therapeutic and well-tolerated dose.
Intellectual Property Related to XEN1101
The company has a comprehensive strategy in place to protect and expand the intellectual property portfolio that covers XEN1101. Importantly, two additional U.S. patents were granted in 2021 covering claims related to: distinct crystalline forms of XEN1101 drug substance and pharmaceutical compositions containing the same as compositions-of-matter, along with methods for their preparation and use; and methods of enhancing the bioavailability of XEN1101 by administration with or close to a meal. These U.S. patents are expected to expire in 2040 and 2039, respectively, absent any extensions of patent term.
XEN496, a Kv7 Potassium Channel Opener
XEN496, a Kv7 potassium channel opener, is a proprietary, pediatric formulation of the active ingredient ezogabine being developed for the treatment of KCNQ2 developmental and epileptic encephalopathy, or KCNQ2-DEE. The Kv7 potassium channel mechanism has been clinically validated with ezogabine, an earlier generation Kv7 opener that was approved by the FDA as an adjunctive treatment for adults with focal seizures with or without secondary generalization. Published case reports where physicians have used ezogabine in infants and young children with KCNQ2-DEE suggest that XEN496 may be efficacious in this often hard-to-treat pediatric patient population.
The company has received Fast Track designation and orphan drug designation, or ODD, for XEN496 for the treatment of seizures associated with KCNQ2-DEE from the FDA, as well as an orphan medicinal product designation from the European Commission. The FDA previously indicated that it is acceptable to study XEN496 in infants and children up to four years old, and that a single, small pivotal trial may be considered adequate in order to demonstrate XEN496’s efficacy in KCNQ2-DEE, provided the study shows evidence of a clinically meaningful benefit in patients with the intended indication.
Clinical Development of XEN496
The company has developed XEN496 as a proprietary, pediatric-specific, immediate-release formulation of ezogabine. To support the Phase 3 clinical trial of XEN496 in patients with KCNQ2-DEE, it completed a PK study testing its pediatric formulation in 24 healthy adult volunteers. The PK profile observed for XEN496 was comparable to historical PK data for immediate-release ezogabine tablets, with XEN496 showing similar absorption and elimination curves.
The company has an ongoing Phase 3 randomized, double-blind, placebo-controlled, parallel group, multicenter clinical trial, called the EPIK study, evaluating the efficacy, safety, and tolerability of XEN496 administered as adjunctive treatment in approximately 40 pediatric patients aged one month to less than 6 years with KCNQ2-DEE. The company anticipates that the EPIK study will be completed in 2024.
KCNQ2-DEE
KCNQ2 developmental and epileptic encephalopathy, or KCNQ2-DEE, otherwise known as EIEE7, is a rare, severe neurodevelopmental disorder with a significant seizure burden and profound developmental impairment. KCNQ2-DEE is uniquely characterized by multiple, daily, refractory seizures presenting within the first week of life with a prominent tonic component and autonomic signs. Seizures are often accompanied by clonic jerking or complex motor behavior. The EEG at onset of the disease shows a burst suppression pattern later evolving into multifocal epileptiform activity. The infants usually develop a severe to profound intellectual disability with axial hypotonia that can be accompanied by limb spasticity. The seizure activity typically decreases with age with some patients becoming seizure free or experiencing more minor seizure burden by 3 to 5 years of age; however, a survey of patient caregivers indicates that a significant proportion of patients have ongoing seizures beyond this age range.
New Pipeline Opportunities
Given its expertise in drug discovery, the company’s efforts are concentrated on the identification of ion channel targets where novel openers might represent significant therapeutic advances, with a particular focus on epilepsy and other CNS-related indications. Expansion of the company’s pipeline may come from its internal research efforts and through the acquisition or in-licensing of other external product candidates.
Partnered Programs
NBI-921352, A Clinical Stage, Selective Nav1.6 Sodium Channel Inhibitor for the Treatment of Epilepsy
In December 2019, the company entered into a license and collaboration agreement with Neurocrine Biosciences to develop treatments for epilepsy. Neurocrine Biosciences has an exclusive license to XEN901, now known as NBI-921352, a clinical stage selective Nav1.6 sodium channel inhibitor, and an exclusive license to pre-clinical compounds for development, including selective Nav1.6 inhibitors and dual Nav1.2/1.6 inhibitors. The agreement also included a multi-year research collaboration to discover, identify and develop additional novel Nav1.6 and Nav1.2/1.6 inhibitors, which was completed in June 2022. Pursuant to the terms of the agreement, the company has the potential to receive certain clinical, regulatory, and commercial milestone payments, as well as future sales royalties.
NBI-921352 is a potent, highly selective Nav1.6 sodium channel inhibitor being developed to treat pediatric patients with SCN8A developmental and epileptic encephalopathy, or SCN8A-DEE, and other potential indications, including adult focal epilepsy. Neurocrine Biosciences has received orphan drug and rare pediatric disease designations from the FDA for NBI-921352 in SCN8A-DEE. Prior to its license and collaboration agreement with Neurocrine Biosciences, the company completed a Phase 1 clinical trial in healthy adult subjects, and subsequently developed a pediatric-specific, granule formulation. Neurocrine Biosciences is conducting a Phase 2 clinical trial evaluating NBI-921352 in adult patients with focal-onset seizures, with data expected in the second half of 2023. In addition, a Phase 2 clinical trial is underway evaluating NBI-921352 in pediatric patients (aged between 2 and 21 years) with SCN8A-DEE.
Collaborations, Commercial and License Agreements
License and Collaboration Agreement with Neurocrine Biosciences, Inc.
On December 2, 2019, the company entered into a license and collaboration agreement, or the Collaboration Agreement, with Neurocrine Biosciences to establish a collaboration under which the parties will identify, research and develop sodium channel inhibitors, including its clinical candidate XEN901, known as NBI-921352, and preclinical candidates XEN393, XPC’535 and XPC’391, which compounds Neurocrine Biosciences will have the exclusive right to further develop and commercialize under the terms and conditions set forth in the Collaboration Agreement.
Under the terms of the Collaboration Agreement the company granted to Neurocrine Biosciences an exclusive, royalty-bearing, sublicensable license to certain of its intellectual property rights for the research, development and commercialization of NBI-921352; XEN393, XPC’535 and XPC’391, collectively referred to as the development track candidates, or the DTCs; and certain research compounds that bind to and inhibit voltage-gated sodium channels Nav1.2 and Nav1.6 as their primary mechanism of action, collectively, the Research Compounds and, together with NBI-921352 and the DTCs, the Compounds, on a worldwide basis for the treatment, cure, diagnosis, prediction or prevention of any human disease or disorder, state, condition and/or malady, subject to certain exceptions set forth in the Collaboration Agreement. The company also granted to Neurocrine Biosciences a non-exclusive, non-royalty-bearing, sublicensable license to certain of its intellectual property rights for the screening of compounds for identification as a Select Nav Inhibitor and for the research of certain compounds otherwise expressly excluded from the Collaboration Agreement, or the Excluded Compounds.
The company is collaborating with Neurocrine Biosciences on the conduct of two collaboration programs: a joint research collaboration to discover, identify and preclinically develop Research Compounds, or the Research Program, and a collaborative development program for NBI-921352 and two DTCs selected by the JSC, or the Initial Development Program and, together with the Research Program, referred to as the Collaboration Programs. The Research Program included the preclinical development of its existing non-clinical Research Compounds and the discovery of new back-up and follow-on Research Compounds to NBI-921352 and the two DTCs selected by the JSC as clinical development candidates for subsequent development and commercialization by Neurocrine Biosciences. The Research Program was completed in June 2022.
Neurocrine Biosciences has the exclusive right to conduct, and will be solely responsible for all aspects of, the commercialization of any pharmaceutical product that contains a Compound.
In January 2021, the company entered into an amendment with Neurocrine Biosciences pursuant to which it revised certain IND acceptance criteria relating to NBI-921352 for the potential treatment of SCN8A-DEE.
In February 2022, the company entered into a second amendment with Neurocrine Biosciences pursuant to which the restrictions imposed on Neurocrine Biosciences prohibiting it from developing, seeking regulatory approval for, marketing or promoting certain early compounds (as the term is defined in the Collaboration Agreement) in the pain field (as the term is defined in the Collaboration Agreement) were removed.
Intellectual Property
As of December 31, 2022, the company owned, co-owned or licensed 30 issued U.S. patents and approximately 18 pending U.S. patent applications, including provisional and non-provisional filings. The company also owned, co-owned or licensed approximately 363 pending and granted counterpart applications worldwide, including 11 country-specific validations of three European patents.
As of December 31, 2022, the company owned four issued U.S. patents and six pending U.S. non-provisional patent applications related to XEN1101, and methods of making and using XEN1101 and certain related compounds. Upon issuance, the patents are expected to expire between 2028 and 2042 (absent any extensions of term). In addition, the company has approximately 15 foreign issued patents (exclusive of European patent national validations), two pending PCT international applications, and approximately 129 pending applications in various foreign jurisdictions relating to XEN1101 and certain related compounds.
As of December 31, 2022, the company had filed one U.S. provisional patent application directed to certain of its potassium channel modulators (exclusive of XEN1101), as well as methods of making and using the same. Any patents issuing from this application are expected to expire in 2043 (absent any extension in term).
As of December 31, 2022, the company had filed one U.S. non-provisional patent application and approximately 32 pending applications in various jurisdictions directed to XEN496 (i.e., its pediatric formulation of ezogabine), a genus of related formulations, and methods of making and using the same. Any patents issuing from this application are expected to expire in 2040 (absent any extensions of term).
As of December 31, 2022, the company owned four issued U.S. patents, one pending U.S. non-provisional patent application, and one U.S. provisional patent application directed to NBI-921352 (formerly known as XEN901) and methods of making and using this and certain related compounds. The issued patents, along with any patents issuing from these applications, are expected to expire between 2037 and 2042 (absent any extensions of term). In addition, the company owned approximately nine foreign issued patents (exclusive of European patent national validations), two pending PCT international applications, and the company had approximately 17 pending corresponding applications in various foreign jurisdictions relating to NBI-921352 and certain related compounds. Pursuant to its collaboration with Neurocrine Biosciences, Neurocrine Biosciences will oversee the prosecution, maintenance and other matters relating to the patent portfolio for NBI-921352 and the other selective Nav1.6 inhibitors and dual Nav1.2/1.6 inhibitors.
As of December 31, 2022, the company owned five issued U.S. patents, and two U.S. non-provisional patent applications directed to certain of its selective inhibitors of Nav1.6 and/or Nav1.2 (exclusive of NBI-921352), as well as methods of making and using the same. The issued patents, along with any patents issuing from these applications are expected to expire between 2037 and 2039 (absent any extensions of term). In addition, the company owned approximately seven foreign issued patents and approximately 97 pending corresponding applications in various foreign jurisdictions (exclusive of European patent national validations) relating to its selective inhibitors of Nav1.6 and/or Nav1.2 (exclusive of NBI-921352), as well as methods of making and using the same.
As of December 31, 2022, the company co-owned one issued U.S. patent directed to Nav1.7 inhibitors, as well as methods of making and using the same. The issued patent is expected to expire in 2036 (absent any extensions of term).
Government Regulation
The company is developing small-molecule product candidates, which are regulated as drugs by the FDA and equivalent regulatory authorities outside the U.S. Within the FDA, the Center for Drug Evaluation and Research, or CDER, regulates drugs. Drugs are subject to regulation under the Federal Food, Drug, and Cosmetic Act, or FD&C Act, and other federal, provincial, state, local and foreign statutes and regulations.
The company also must comply with the FDA’s advertising and promotion requirements, such as those related to direct-to-consumer advertising, the prohibition on promoting products for uses or in patient populations that are not described in or are otherwise inconsistent with the product’s approved labeling (known as off-label use), and industry-sponsored scientific and educational activities.
In the U.S., the research, manufacturing, distribution, sale and promotion of drug products that the company is developing are subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other state and local government agencies.
The U.S. Foreign Corrupt Practices Act and the Canadian Corruption of Foreign Public Officials Act, the U.S. Travel Act, the OECD Anti-Bribery Convention, Title 18 United States Code section 201, and any other applicable domestic or foreign anti-corruption or anti-bribery laws to which the company is subject prohibit corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity.
History
The company was founded in 1996. It was incorporated in the province of British Columbia in 1996 under the name Xenon Bioresearch Inc. and changed its name to Xenon Genetics Inc. in 2000. Further, the company changed its name to Xenon Pharmaceuticals Inc. in 2004.

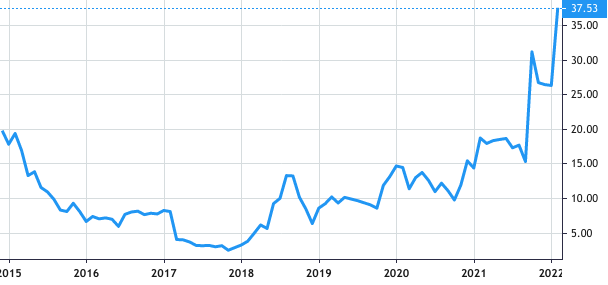
 Stock Value
Stock Value

