About Tenax Therapeutics
Tenax Therapeutics, Inc. engages in developing and planning to commercialize pharmaceutical products containing imatinib for the treatment of pulmonary arterial hypertension (PAH).
The company has been developing TNX-103 (oral levosimendan) for the treatment of Pulmonary Hypertension with Heart Failure with Preserved Ejection Fraction (PH-HFpEF) and TNX-201 (modified release imatinib) for the treatment of pulmonary arterial hypertension (PAH). Both TNX-103 and TNX-201 are Phase 3-ready assets, each with the potential to meaningfully impact the quality and longevity of patient lives.
On November 13, 2013, the company acquired a license granting Life Newco, its wholly-owned subsidiary, an exclusive, sublicensable right to develop and commercialize pharmaceutical products containing levosimendan, 2.5 mg/ml concentrate for solution for infusion / 5ml vial in the United States and Canada. On October 9, 2020 and January 25, 2022, the company entered into an amendment to the license to include two new oral products containing levosimendan, in capsule and solid dosage form, and a subcutaneously administered product containing levosimendan, subject to specified limitations.
Strategy
The key elements of the company's strategy include efficiently conducting clinical development to establish clinical proof of principle in new indications, refine formulation, and commence Phase 3 testing of its product candidates; efficiently exploring new high-potential therapeutic applications, in particular where expedited regulatory pathways are available, leveraging third-party research collaborations and its results from related areas; continuing to expand its intellectual property portfolio; entering into licensing or product co-development arrangements.
Programs

TNX-101 (IV), TNX-102 (subcutaneous) and TNX-103 (oral) ( (levosimendan)
Levosimendan is a calcium sensitizer/K-ATP activator developed for intravenous use in hospitalized patients with acutely decompensated heart failure. In 2013, the company acquired certain assets of Phyxius Pharma, Inc. (Phyxius), including its North American rights to develop and commercialize intravenous levosimendan for any indication in the United States and Canada. The license was subsequently amended in 2020 to include the rights to develop and commercialize oral and subcutaneous formulations of levosimendan.
In 2020, the company completed a Phase 2 clinical trial of intravenous levosimendan in North America. In March 2018, the company met with the United States Food and Drug Administration (FDA) to discuss development of levosimendan in these patients. The FDA agreed with the company's planned Phase 2 design, patient entry criteria, and endpoints. The company initiated the first of its HELP Study clinical sites in November 2018 and the first of 37 patients was enrolled in the HELP Study in March 2019. Enrollment in the HELP Study was completed about one year later, in March 2020. On June 2, 2020, the company announced preliminary, top-line data from the study.
The detailed results from the Phase 2 HELP Study of levosimendan in preserved ejection fraction and associated pulmonary hypertension (PH-HFpEF) were presented at the Heart Failure Society of America Virtual Annual Scientific Meeting on October 3, 2020 and at the American Heart Association Scientific Sessions 2020 on November 13, 2020.
On October 9, 2020, the company entered into an Amendment to the License Agreement between the company and Orion to include two new product formulations containing levosimendan, in a capsule solid oral dosage form (TNX-103) and a subcutaneously administered dosage form (TNX-102), to the scope of the license, subject to specified limitations. On January 4, 2022, the company was issued U.S. Pat. No. 11,213,524, entitled PHARMACEUTICAL COMPOSITIONS FOR SUBCUTANEOUS ADMINISTRATION OF LEVOSIMENDAN.
The company and its HELP investigators continued studying the safety and efficacy of TNX-103 in all patients participating in the open-label extension of the HELP Study, all of whom previously received weekly infusions of intravenous levosimendan. The company expects the open-label extension study (OLE) to come to a conclusion in the first half of 2023.
TNX-201 (imatinib)
Imatinib (marketed in the U.S. as Gleevec) is a tyrosine kinase inhibitor, which changed the treatment of chronic myeloid leukemia (CML) following its approval over 20 years ago, as the first curative treatment of chronic leukemia.
On May 30, 2019, PHPrecisionMed Inc., a Delaware corporation (PHPM), which was to be acquired by the company in January 2021, met with the FDA to discuss a proposal for a Phase 3 trial of imatinib for PAH.
Phase 3 study of TNX-201, this optimized modified release formulation of imatinib, would be the next clinical trial to commence in the company's development planning, pending the outcome of its strategic process.
Manufacturing and Supply
Pursuant to the terms of the company's license for levosimendan, Orion Corporation (Orion) is contractually its sole manufacturing source for TNX-103. The company has engaged various third-party suppliers and contract manufacturing organizations (CMOs) for the supply and manufacture of imatinib for potential future clinical trials, and relied on such contractors for material contributing to TNX-201, for testing in its two completed Phase 1 trials.
Intellectual Property
The company has two granted patents, and two U.S. patent applications pending, related to product candidates and proprietary process, method and technology. The company's issued levosimendan patents expire in 2039 and late 2040.
On January 4, 2022, the company received a patent for the subcutaneous administration of levosimendan, whether through the formulation it has developed in collaboration with a formulation development partner, or other subcutaneous formulations meeting certain broad characteristics defined in the patent. In addition, the company received on March 21, 2023, a patent for the use of IV levosimendan in the treatment of PH-HFpEF patients, based on several discoveries that have emerged from the HELP Study and the OLE.
The U.S. trademark registration for Simdax is owned by Orion and is licensed to the company for sales and marketing purposes for any intravenous pharmaceutical products containing levosimendan that are commercialized in the United States and Canada.
Simdax License Agreement
On November 13, 2013, the company acquired, through its wholly-owned subsidiary, a license agreement between Phyxius and Orion, which was later amended on October 9, 2020 and January 25, 2022 (as amended, the License). The License grants the company an exclusive, sublicenseable right to develop and commercialize pharmaceutical products containing levosimendan in the United States and Canada (the Territory) and, pursuant to the October 9, 2020 amendment to the License, also includes two product dose forms containing levosimendan, in capsule and solid dosage form, and a subcutaneously administered product containing levosimendan, subject to specified limitations (together, the Product). Pursuant to the License, the company and Orion will agree to a new trademark when commercializing levosimendan in either of these forms.
Orion's ongoing role under the License includes sublicense approval, serving as the sole source of manufacture of oral formulations of levosimendan, holding a first right to enforce intellectual property rights in the United States and Canada, and certain regulatory participation rights. Orion must notify the company before the end of 2024 if it chooses not to exercise its right to supply oral formulations of levosimendan to the company for commercialization in the Territory. Additionally, the company must grant back to Orion a broad non- exclusive license to any patents or clinical trial data related to levosimendan developed by the company under the License. The term of the License extends until 10 years after the launch of a levosimendan product in the United States and Canada, provided that the License will continue after the end of the term in each country in the Territory until the expiration of Orion's patent rights in levosimendan in such country. In the event that no regulatory approval for levosimendan has been granted in the United States on or before September 20, 2030, however, either party will have the right to terminate the License with immediate effect.
Research and Development Expenses
The company's research and development expenses were $5.4 million for the year ended December 31, 2022.
Government Regulation
The Federal Food, Drug and Cosmetic Act and the Public Health Service Act govern the testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion of the company's medical products. In addition to FDA regulations, the company is subject to other federal and state regulations, such as the Occupational Safety and Health Act and the Environmental Protection Act.
History
The company was founded in 1967. The company was incorporated as a New Jersey corporation in 1967. It was formerly known as Synthetic Blood International, Inc. and changed its name to Oxygen Biotherapeutics, Inc., and changed the domiciliary state of the corporation to Delaware in 2008. Further, the company changed its name to Tenax Therapeutics, Inc. in 2014.

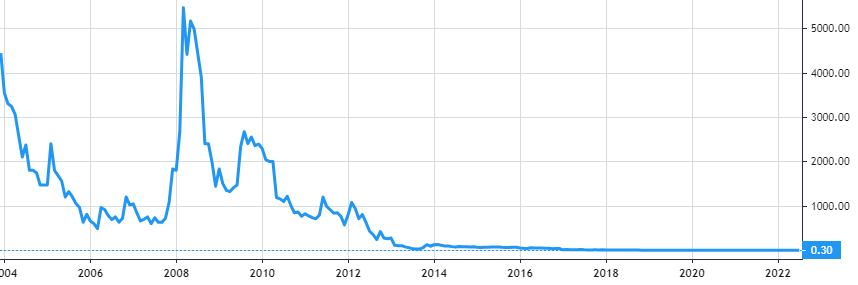
 Stock Value
Stock Value

