About Sintx Technologies
SINTX Technologies, Inc. (SINTX) operates as an advanced materials company that develops and commercializes advanced ceramics for biomedical, industrial, and antipathogenic applications. The company focuses on the manufacturing, research, and development of advanced ceramics for external partners.
In June 2022, the company acquired Technology Assessment and Transfer, Inc. (TA&T), a nearly 40-year-old business with a mission to transition advanced materials and process technologies from a laboratory environment to commercial products and services. TA&T has supplied ceramics for use in several biomedical applications. These products were made via 3D printing and include components for surgical instruments, as well as conceptual and prototype dental implants.
Products
Silicon Nitride
The company operates its own silicon nitride manufacturing facility. Its 30,000 square foot corporate facility includes an 18,000 square foot FDA registered ISO 13485:2016 certified, and AS9100D certified manufacturing space. It is equipped with state-of-the-art powder processing, spray drying, pressing and computerized machining equipment, sintering furnaces, and other testing equipment that enables the company to control the entire manufacturing process for its silicon nitride products and product candidates. All operations with the exception of raw material production are performed in-house.
The company produces silicon nitride for use in its commercial products and product candidates in the following forms:

Solid Silicon Nitride: This form of silicon nitride is a fully dense, load-bearing solid, which can be used for devices that require high strength, toughness, fracture resistance, and low wear. Applications include medical devices, such as interbody spinal fusion implants; and non-medical, such as electrical and aerospace components.
Porous Silicon Nitride: While this form of silicon nitride has a chemical composition that is identical to that of the company's monolithic solid silicon nitride, this formulation has a porous structure, which is engineered to mimic cancellous bone, the spongy bone tissue that typically makes up the interior of human bones. The company's porous silicon nitride has interconnected pores ranging in size between about 90 and 600 microns, which is similar to that of cancellous bone. This form of silicon nitride can be used for the promotion of bone in-growth and attachment.
Silicon Nitride Powder: The company can produce silicon nitride powder that is osteogenic and antipathogenic. This powder can then be utilized to produce composites or coatings.
Composite of Silicon Nitride and PEEK: The company has demonstrated in the laboratory that it is possible to compound its silicon nitride powder and the polymer PEEK and that the ensuing composite material maintains the bioactive properties of silicon nitride. It has engaged academic and commercial partners to assist it in developing this technology and has received an NIH grant to assist in advancing this work. This composite material would allow the straightforward machinability of a complex device that would be more challenging to manufacture from silicon nitride alone.
Silicon Nitride Coating: With a similar chemical composition as the company's other forms of silicon nitride, this form of silicon nitride can be applied as an adherent coating to metallic substrates, including cobalt-chromium, titanium and steel alloys, polymers, and ceramics. The use of silicon nitride coating may also create an antibacterial, antiviral, and antifungal barrier between the device and the adjacent bone or tissue. The company is evaluating several different coating technologies.
Other Advanced Ceramic Products
Ceramic Armor
In 2021, SINTX entered the ceramic armor market through the purchase of assets from B4C, LLC, Dayton, Ohio, and a technology partnership with Precision Ceramics USA. The company operates its armor business through its wholly owned subsidiary SINTX Armor, Inc. It will develop and manufacture high-performance ceramics for personnel, aircraft, and vehicle armor including a 100% boron carbide material for ultimate lightweight performance in ballistic applications, and a composite material made of boron carbide and silicon carbide for exceptional multi-hit performance against ballistic threats. Armor solutions utilizing ceramics are commonly used to protect vehicles, personnel, aircraft, and marine vessels due to their light weight and high hardness.
Boron carbide has additional uses, including wear components, such as nozzles, and as a neutron absorber in nuclear reactors. SINTX is pursuing opportunities in these market segments as well.
The company has signed a 10-year lease at a building near its headquarters in Salt Lake City, Utah to house development and manufacturing activities for SINTX Armor. The company has relocated the B4C (B4C, LLC) assets from Dayton into this facility and has made necessary upgrades to the facility infrastructure to operate the equipment.
Strategy
The key elements of the company's strategy include to develop new products with anti-pathogenic properties, including the inactivation of the SARS-CoV-2 virus, utilizing its silicon nitride technology; develop additional commercial opportunities outside of the medical device market; develop new silicon nitride manufacturing technologies; and apply its silicon nitride technology platform to new medical opportunities.
Intellectual Property
The company has eleven issued U.S. patents, five foreign patents, eighteen pending U.S. non-provisional patent applications, no pending U.S. provisional patent applications, eighty-five pending foreign applications and six pending PCT patent applications. Its first issued patent expired in 2016, with the last of these patents expiring in 2039.
The company has three U.S. patents directed to articulating implants using its high-strength, high toughness doped silicon nitride solid ceramic. These issued patents, which include U.S. 7,666,229; U.S. 9,051,639; and U.S. 9,517,136 will expire in November 2023, September 2032, and March 2034, respectively.
The company also has one U.S. patent related to its CSC technology that are directed to implants that have both a dense load-bearing, or cortical, component and a porous, or cancellous, component, together with a surface coating. The issued patent U.S. 9,649,197 will expire in July 2035.
In addition, U.S. Patent No. 10,806,831 directed to antibacterial implants and U.S. Patent No. 11,191,787 directed to antipathogenic devices were recently issued which will expire in 2037 and 2039, respectively.
With respect to PCT patent application serial no. PCT/US2018/014781 directed to antibacterial biomedical implants, the company entered the national stage in Europe, Australia, Brazil, Canada, China, Japan, Hong Kong, and South Korea, as well as one divisional patent application filed in Europe and two divisional applications filed in Japan to seek potential patent protection for its proprietary technologies in those countries.
With respect to PCT patent application serial no. PCT/US2019/026789 directed to methods for improving the wear performance of ceramic-polyethylene or ceramic-ceramic articulation couples utilized in orthopaedic joint prostheses, the company entered the national stage in Australia, Brazil, Canada, Europe, Japan, Korea, and Mexico to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2019/048072 directed to antipathogenic devices and methods, the company entered the national stage in Europe, Japan, Mexico, Australia, Brazil, Canada, South Korea, China, and India to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2020/037170 directed to methods of surface functionalization of zirconia-toughened alumina with silicon nitride, the company entered the national stage in Europe, Australia, Brazil, Canada, China, India, Japan, and Mexico to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2021/014725 directed to antifungal composites and methods thereof, the company entered the national stage in Europe, Brazil, Japan, Australia, Canada, China, India, Mexico, and South Korea to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2021/027258 directed to antipathogenic face mask, the company entered the national stage in Australia, Brazil, Canada, China, Europe, India, Japan, South Korea, and Mexico to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2021/027263 directed to systems and methods for rapid inactivation of SARS-CoV2 by silicon nitride, copper, and aluminum nitride, the company entered the national stage in Australia, Brazil, Canada, China, Europe, India, Japan, South Korea, and Mexico to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2021/038364 directed to antipathogenic devices and methods thereof for antifungal applications, the company entered the national stage in Australia, Brazil, Canada, China, Europe, India, Japan, South Korea, and Mexico to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no. PCT/US2021/028975 directed to methods for laser coating of silicon nitride on a metal substrate, the company entered the national stage in Australia, Brazil, Canada, China, Europe, India, Japan, South Korea, and Mexico to seek patent protection for its proprietary technologies in those countries.
With respect to PCT application serial no PCT/US2021/028641 directed to methods of silicon nitride laser cladding, the company entered the national stage in Australia, Brazil, Canada, China, Europe, India, Japan, South Korea, and Mexico to seek patent protection for its proprietary technologies in those countries.
In relation to the sale of the company's spine implant business to CTL Medical under the Asset Purchase Agreement dated September 5, 2018, it assigned its entire right to forty-eight U.S. patents, two foreign patents and three pending patent applications from its patent portfolio to CTL Medical under that transaction. In addition, three U.S. patents (U.S. patent nos. 9,399,309; 9,517,136; and 9,649,197) directed to silicon nitride manufacturing processes were licensed to CTL Medical under an irrevocable, fully paid-up, worldwide license for a ten-year term with CTL Medical also having a Right of First Negotiation to acquire these patents if SINTX decides to later sell these IP assets to a third party.
Competition
The company's main competitors in the industrial market segment include CoorsTek, Kyocera, and Saint Gobain. Its main competitors in the antipathogenic market segment include BactiGuard and MicroBan.
History
The company was founded in 1996. It was incorporated in 1996. The company was formerly known as Amedica Corporation and changed its name to Sintx Technologies, Inc. in 2018.
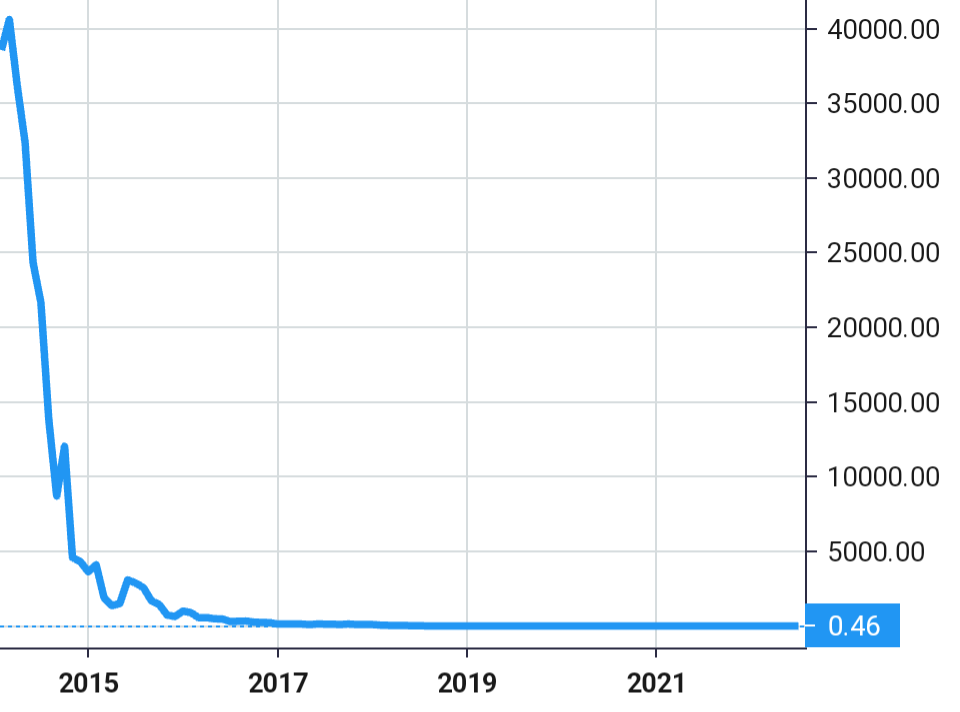
 Stock Value
Stock Value

