About Altamira Therapeutics
Altamira Therapeutics Ltd. operates as a clinical-and commercial-stage biopharmaceutical company. The company is developing therapeutics that address important unmet medical needs.
The company is active in two areas: the development of RNA delivery technology and therapeutics for extrahepatic targets (OligoPhore / SemaPhore platforms; AM-401 for the treatment of KRAS driven cancer, AM-411 for the treatment of rheumatoid arthritis; preclinical), and nasal sprays for protection against airborne allergens, and where approved, viruses (Bentrio; commercial) or the treatment of vertigo (AM-125; Phase 2). The company has announced its intention to reposition its around RNA delivery technology while exploring strategic options to either divest its non-RNA traditional businesses or partner them with one or several other companies. In particular, the company has announced that it is in active discussions for the partnering of Bentrio for North America, Europe, and other key markets and has been active in the process of selling its inner ear therapeutics assets.
OligoPhore / SemaPhore platforms
Through the acquisition of Trasir in June 2021, the company entered the field of RNA delivery technology. Trasir's core technology is the proprietary peptide polyplex platform OligoPhore and its equivalent SemaPhore that can engage any type of short interfering RNA (siRNA) or messenger RNA (mRNA), respectively, in rapid self-assembly.
AM-401
In July 2021, the company announced the selection of KRAS-driven cancer as the first therapeutic indication for its OligoPhore oligonucleotide delivery platform. The company started in silico and in vitro work to screen and select the most effective siRNA sequences and to optimize their properties. The company intends to review and discuss its plans for IND-enabling preclinical studies with the FDA in the context of a Pre-IND meeting. The company expects to conduct a Phase 1 clinical trial in patients with KRAS driven cancer.

AM-411
In 2022, the company announced the initiation of AM-411, its second development project for an RNA therapeutic based on the OligoPhore delivery platform. AM-411 seeks to treat rheumatoid arthritis (RA) by targeting siRNA at p65, one of the main transcriptional regulators of the NF-kB pathway and a key checkpoint in RA inflammation.
AM-411 is a polyplex nanoparticle delivering siRNA to inflamed tissues to target the NF-kappaB signaling pathway, a critical regulator of immune and inflammatory responses. Like AM-401, the drug product is based on its OligoPhore technology which allows for delivery of RNA payloads specifically to inflamed tissues with extensive endosomal release once inside cells, generating a new class of precision medicines with potentially increased local efficacy and reduced systemic side effects. AM-411 consists of an optimized siRNA targeting p65, one of the main transcriptional regulators of the NF-kB pathway and a key checkpoint in RA inflammation that has generated high interest as a target.
AM-125
The company is developing AM-125 for the intranasal treatment of acute vestibular syndrome (AVS). In 2017, the company entered into an asset purchase agreement with Otifex Therapeutics Pty Ltd (Otifex), pursuant to which it has purchased various assets related to betahistine dihydrochloride in a spray formulation, which it has been developing for intranasal treatment of vertigo under the product code AM-125.
In 2018, the company conducted a second Phase 1 clinical trial with AM-125 in 72 healthy volunteers.
In June 2022, the company announced top-line data from its Phase 2 TRAVERS trial with AM-125 in AVS. The company has discussed the regulatory requirements for AM-125 during a pre-Investigational New Drug meeting with the FDA and in the context of scientific advice meetings with the EMA and two European national health authorities to further define the development program. The company has designed additional trials with AM-125 and in May 2023 submitted an application for an IND to the FDA. As part of its strategic repositioning, the company expects to either divest the AM-125 program or out-license it.
Under product code AM-201, the company evaluated intranasal betahistine also for its potential use in the prevention of antipsychotic induced weight gain and drowsiness. In 2019, the company initiated a Phase 1b trial in Europe to evaluate AM-201's safety and therapeutic effects in this indication.
Bentrio (AM-301)
In 2020, the company announced the launch of the development of Bentrio (AM-301), a drug-free nasal spray for protection against airborne viruses and allergens through a newly created subsidiary, Altamira Medica Ltd. Bentrio, which is being commercialized in Germany and other European countries under the CE mark as an over the counter product under the brand name Bentrio, is a gel emulsion which works by forming a protective layer on the nasal mucosa that acts as a mechanical barrier against airborne viruses allergens. The barrier consists of two elements, a mucoadhesive film lining the nasal cavity and preventing contact of airborne viruses or allergens with the nasal mucosa to reduce the risk of viral infection or allergic reactions; and the trapping / binding of such viruses or allergens through electrostatic effects, allowing for their removal e.g. through mucociliary clearance. In addition, the product helps to humidify and thus maintain the nasal mucosa's function in clearing viruses and allergens from the nasal cavity. The key component of Bentrio is bentonite, a naturally occurring clay.
The company has shown that Bentrio provides help to people suffering from allergic rhinitis by reducing their exposure to airborne allergen particles e.g. from pollens, house dust or animal hair. In 2021, the company conducted an open-label randomized cross-over study with AM-301 that enrolled 36 patients with allergic rhinitis caused by grass pollen.
In May 2022, the company announced the results from a clinical trial with Bentrio in house dust mite (HDM) allergic rhinitis.
In December 2022, the company completed enrollment into a randomized controlled clinical trial (the 'NASAR' trial) with Bentrio in seasonal allergic rhinitis (SAR) in Australia. In January 2023, the company announced the results of an interim analysis based on data from the first 53 participants in the NASAR trial.
The company tested Bentrio in vitro in a series of experiments using reconstituted human nasal epithelia infected with SARS-CoV-2 or H1N1 influenza virus. In January 2023, the company announced top-line data from a clinical trial in acute COVID-19 ('COVAMID').
In 2021, the company completed the conformity assessment procedure for marketing the product in the member states of the European Union (EU).
In 2021, the company filed a 510(k) premarket notification with the FDA, for premarket clearance of Bentrio as a Class II device for the intended use of promoting alleviation of mild allergic symptoms triggered by the inhalation of various airborne allergens. In 2022, the company received 510(k) clearance for Bentrio.
The company intends to market Bentrio primarily through third parties. The company already has entered into marketing and distribution agreements with several collaboration partners in Asia and Europe and intends to cover further markets in particular in Europe and North America.
Keyzilen
The company has been developing Keyzilen, Esketamine gel for injection, for the treatment of acute inner ear tinnitus. Esketamine is a potent, small molecule non-competitive NMDA receptor antagonist. Keyzilen is formulated in a biocompatible gel and delivered via intratympanic injection.
In 2019, the company announced that it had completed the design of a pivotal Phase 2/3 trial for Keyzilen. In 2019, the company announced that it has obtained advice on the development plan and regulatory pathway from the U.S. Food and Drug Administration (FDA) in the context of a Type C meeting and from the European Medicines Agency (EMA) in the context of a Scientific Advice procedure for Keyzilen.
Sonsuvi
The company also has been developing Sonsuvi for acute inner ear hearing loss. In a Phase 2 clinical trial, AM-111 showed a favorable safety profile. Furthermore, in patients with severe to profound ASNHL, it observed a clinically relevant improvement in hearing threshold, speech discrimination and a higher rate of complete tinnitus remission compared with placebo. In November 2017, the company announced that the HEALOS Phase 3 clinical trial that investigated Sonsuvi in the treatment of acute inner ear hearing loss did not meet the primary efficacy endpoint.
Based on the HEALOS results, the company submitted the design of a new pivotal trial with AM-111 0.4 mg/mL in patients suffering from acute profound hearing loss to the European Medicines Agency (EMA) and subsequently also to the FDA for review. In the context of the company's strategic repositioning, it aims to divest or partner the Sonsuvi program.
Strategy
The company's proprietary peptide polyplex platform OligoPhore and its equivalent SemaPhore can engage any type of short interfering RNA (siRNA) or messenger RNA (mRNA), respectively, in rapid self-assembly.
The key elements of the company's strategy are to demonstrate preclinical and clinical proof of concept with OligoPhore in two lead indications; leverage OligoPhore/ SemaPhore platform through partnering; and focus activities on RNA delivery technology by divesting or partnering its non-RNA businesses.
Intellectual Property
Patents
As of December 31, 2022, the company owned ten issued U.S. patents and five pending U.S. patent applications along with foreign counterparts of particular patents and applications in various jurisdictions. The company co-owns four of its issued U.S. patents along with their foreign counterparts, pursuant to the terms of its co-ownership and exploitation agreement with INSERM.
Bentrio
In September 2021, the company converted four provisional US patent filings into a non-provisional US patent application relating to the formulation and use of Bentrio. In addition, the company filed an international Patent Cooperation Treaty (PCT) patent application.
Intranasal Betahistine
The company has acquired from Otifex a patent application on the composition and use of intranasal betahistine, which issued on October 29, 2019, as a US patent covering the composition and use of intranasal betahistine. Further, the company acquired in 2018 two U.S. patents relating to the use of betahistine for the prevention and treatment of olanzapine induced weight gain, and it acquired in 2019 two U.S. patents relating to the use of betahistine for the treatment of attention deficit/hyperactivity disorder and atypical depression.
AM-401 and AM-411
The company is the exclusive licensee under its agreement with Washington University of a portfolio of patents and patent applications that relate to peptide based polyplexes for RNA delivery. The portfolio includes two issued US patents along with their foreign counterparts in various jurisdictions that cover the composition of matter or method of use of the peptide based polyplexes. These licensed patents and patent applications relating to AM-401, AM-411 and potential further applications of the technology are expected to expire between 2034 and 2037, prior to any patent term extensions. In February 2023, the company filed a provisional patent application with the USPTO describing novel nanoparticle compositions based on OligoPhore or derivatives thereof in combination with siRNA sequences designed to silence different types rather than one specific type of mutated KRAS (polyKRASmut). If granted, the new patent would be expected to expire in 2043.
Keyzilen
The company is the owner or co-owner of patents and patent applications relating to Ketamine or its use in inner ear tinnitus. In particular, the company has an agreement entitled Co-Ownership/Exploitation Agreement with INSERM with respect to its Ketamine patent portfolio. The company has rights to four issued U.S. patents and corresponding patents and applications in other jurisdictions covering formulation and use of Ketamine. The company's issued patents relating to Keyzilen are expected to expire between 2024 and 2028.
Sonsuvi
The company is the exclusive licensee under its agreement with Xigen of a portfolio of patents that relate, among other things, to JNK ligand peptides or their use in hearing loss. This portfolio includes seven issued U.S. patents along with their foreign counterparts in various jurisdictions that cover the composition of matter or method of use of the JNK ligand peptides. These licensed patents and patent applications relating to Sonsuvi are expected to expire between 2023 and 2027, prior to any patent term extensions to which the company may be entitled under applicable laws.
Proprietary Rights
The company has obtained orphan drug designation for Sonsuvi for the treatment of ASNHL in the United States and Europe. In addition, the company has acquired a U.S. orphan drug designation for betahistine for the treatment of obesity associated with Prader-Willi syndrome.
The company has obtained U.S. trademark registrations for Altamira, Auris Medical Cochlear Therapies (and Design), Keyzilen and Sonsuvi. Further, the company has obtained several U.S. trademark registrations for betahistine.
Collaboration and License Agreements
Washington University
On December 11, 2020, the company entered into an Exclusive License Agreement with Washington University, which Exclusive License Agreement was subsequently amended and restated in June 2021 (as so amended and restated, the Agreement), with effect as of December 11, 2020. Pursuant to the Agreement, WU granted the company an exclusive, worldwide, royalty-bearing license (with the right to sublicense) during the term of the Agreement under certain patent rights owned or controlled by WU to research, develop, make, have made, sell, offer for sale, use and import pharmaceutical products covered under such patent rights for all fields of use.
In particular, the company is required to use commercially reasonable efforts to meet the following development milestones to file an IND (or regulatory equivalent in foreign jurisdiction) June 30, 2023; to complete a Phase 1 clinical trial 3.5 years after achieving the first milestone; and to complete a Phase 2 clinical trial four years after achieving the second milestone.
INSERM
In 2006, the company entered into a co-ownership/exploitation agreement with the INSERM, a publicly funded government science and technology agency in France. Pursuant to the terms of the agreement, it was granted the exclusive right to exploit any products derived from patents that resulted from the company's joint research program with INSERM that was conducted in 2003 to 2005 and led to the development of Keyzilen. Pursuant to the terms of the co-ownership/exploitation agreement, the company is given the exclusive right to exploit the patents issuing from the filed patent applications for all claimed applications, including the treatment of tinnitus, in order to develop, promote, manufacture, cause to be manufactured, use, sell and distribute any products, processes or services deriving from such patents, including Keyzilen, in any country in which these patent applications have been filed during the term of the agreement.
As consideration for the exclusive rights granted to the company under the agreement, it has agreed to pay INSERM a two tiered low single digit royalty on the net sales of any product covered by the patents (including the use of Keyzilen in the treatment of tinnitus triggered by cochlear glutamate excitotoxicity) earned in each country in which these patent applications have been filed during the term of the agreement. The company has also agreed to pay INSERM a low double digit fee on any sums of any nature (except royalties and certain costs) collected by it in respect of the granting of licenses to third parties.
Xigen
In October 2003, the company entered into a collaboration and license agreement with Xigen SA (Xigen), pursuant to which Xigen granted the company an exclusive worldwide license to use specified compounds to develop, manufacture and commercialize pharmaceutical products, as well as drug delivery devices and formulations for local administration of therapeutic substances to the inner ear for the treatment of ear disorders (the Area). The company also has a right of first refusal to license certain additional compounds developed by Xigen, which may be used for the Area, specifically any cell permeable inhibitors to effectively block certain signal pathways in apoptotic processes.
Xigen is responsible for maintaining the patents licensed to the company under its agreement. The company retains all know-how and other results from its development of compounds licensed under the agreement.
Research and Development
For the year ended December 31, 2022, the company's research and development expenses included CHF 19.7 million.
History
The company was founded in 2003. It was formerly known as Auris Medical Holding Ltd. and changed its name to Altamira Therapeutics Ltd. in July 2021.
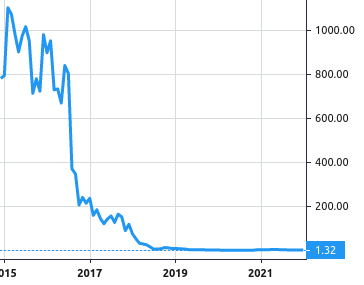
 Stock Value
Stock Value

