About Bellicum Pharmaceuticals
Bellicum Pharmaceuticals, Inc. operates as a biopharmaceutical company.
The company has discovered and developed novel, controllable cellular immunotherapies for various forms of cancer, including both hematological cancers and solid tumors. The company's proprietary Chemical Induction of Dimerization, or CID, technology platform is designed to enable control of components of the immune system in real time. By incorporating the company's CID platform into cellular immunotherapies, the company may enhance their efficacy and safety.
In March 2023, the company announced its decision to discontinue its ongoing Phase 1/2 clinical trials evaluating the safety and preliminary efficacy of the company's GoCAR-T cell product candidates (including BPX-601 and BPX-603) in combination with rimiducid in heavily pre-treated cancer patients following the company's assessment of the risk/benefit profile of BPX-601 in combination with rimiducid.
The company developed GoCAR product candidates the company advanced to Phase 1/2 clinical trials:
BPX-601 is an autologous GoCAR-T product candidate containing the company's proprietary iMC activation switch, designed to treat solid tumors expressing prostate stem cell antigen, or PSCA.
BPX-603 is an autologous dual-switch GoCAR-T product candidate containing both the iMC activation and CaspaCIDe safety switches. BPX-603 is designed to target solid tumors that express the human epidermal growth factor receptor 2 antigen, or HER2.

Product Candidates
BPX-601: GoCAR-T for PSCA+ Solid Tumors
The company was previously developing BPX-601, an autologous GoCAR-T product candidate containing the company's proprietary iMC activation switch, designed to treat solid tumors expressing PSCA. PSCA is an antigen expressed in several solid tumor indications, including prostate and pancreatic cancer. Pre-clinical data show iMC enhances T cell proliferation and persistence, enhances host immune activity, and modulates the tumor microenvironment to improve the potential to treat solid tumors compared to traditional CAR-T therapies.
In March 2023, the company announced its decision to discontinue its ongoing Phase 1/2 clinical trial, called BP-012, in patients with metastatic castration-resistance prostate, or mCRPC; and has begun evaluating strategic alternatives for the company. The most recent patient treated in the Phase 1/2 trial of BPX-601 in mCRPC experienced serious immune-mediated adverse events, including Grade 4 CRS, the second dose-limiting toxicity observed in this cohort of dose escalation. After conducting a thorough review of the risk/benefit observed in BPX-601 as of December 31, 2022, the company determined that, while clinically meaningful efficacy has been observed, the company has the necessary resources to optimize either the clinical dose and schedule of BPX-601 cells and the activating agent rimiducid, or the design of the BPX-601 cell construct to achieve a favorable risk/benefit profile.
The company completed cell dose escalation and lymphodepletion optimization and initiated rimiducid dose escalation in the company's BP-012 study in patients with metastatic pancreatic cancer. The company discontinued enrollment of pancreatic cancer patients in 2021 to focus the company's study of BPX-601 in mCRPC.
In February 2023, the company presented early Phase 1 results at the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) in San Francisco and virtually.
BPX-603: Dual-Switch GoCAR-T for HER2+ Solid Tumors
The company was previously developing BPX-603, which is the company's first controllable dual-switch autologous GoCAR-T product candidate and incorporates both the iMC activation switch and the CaspaCIDe safety switch. BPX-603 is designed to target solid tumors that express the human epidermal growth factor receptor 2 antigen, or HER2. HER2 is a validated antigen for cancer therapies, and academic HER2 CAR-T cell clinical studies have shown evidence of anti-tumor activity. These academic HER2 CAR-T approaches targeting HER2 have been limited by modest clinical efficacy and off-tumor/on-target toxicity. The company's dual-switch GoCAR-T technology may be uniquely suited to improve upon these earlier efforts, by driving greater efficacy through iMC activation while enabling clinicians to manage any treatment-emergent toxicities with CaspaCIDe.
In March 2023, the company announced its decision to discontinue its ongoing Phase 1/2 clinical trial, called BPX603-201A, in patients with metastatic HER2+ solid tumors as discussed above.
Manufacturing, Processing and Delivering to Patients
The company developed efficient and scalable processes to manufacture genetically modified T cells of high quality.
The company's product candidates require a combination of three critical components: viral vectors with DNA content encoded for the company's proprietary switch proteins and co-stimulatory and other accessory molecules; patient-derived T cells that are genetically modified by the company's viral vectors; and the small molecules rimiducid and/or temsirolimus, which activate the switch proteins. Each of these components requires a separate supply chain and shares the same regulatory requirements applicable for biological or chemical materials suitable for human use. Details on each of these components are described below:
Viral Vectors: The company used gamma retrovirus to transduce the company's product candidates. Gamma retrovirus is optimal for cell transduction given that it is an integrating vector that induces long-term gene expression, exhibits high transduction efficiency, has sufficient capacity for DNA content, and has been extensively and safely used in clinical trials.
Genetically Modified Cells: The company has designed and refined a proprietary process for cell engineering that has been improved from lab-based open procedures used in academic and research settings to a functionally closed system that is more appropriate for large-scale clinical trials and commercialization.
Small Molecules: Rimiducid is a synthetic small molecule that has been rationally designed to trigger the proprietary switch proteins in the company's CID platform. The company has separate third-party manufacturers for the active pharmaceutical ingredient, or API, and the finished drug product. Manufacturers of both the API and finished drug product have been selected based on their technical expertise and ability to provide supplies for the company's clinical trial. In its dual-switch constructs, the small molecule temsirolimus can be used to trigger one of the two switches. Temsirolimus is an approved and commercially available product manufactured and distributed by Pfizer Inc. under the trade name TORISEL.
Before the company discontinued its clinical development programs, the company was focused on refining its overall cell therapy supply chain, manufacturing, processing and delivery to patients to be more efficient. The company's process cycles for its autologous product candidates, from collection of white blood cells to infusion of the final product, can be completed in as little as four weeks and are customized to be complementary to the treatment procedure of interest in order to prevent delays or complications.
Intellectual Property
As of December 31, 2022, to the company's knowledge, the company's patent estate, on a worldwide basis, included 192 issued patents, 26 of which are in the U.S., and 63 pending patent applications, 17 of which are in the U.S., which the company owns or for which the company has an exclusive, either in its entirety or within the company's field of use, commercial license. The provisional and pending patent applications and issued patents include composition of matter and method of use claims.
The company has internally developed technology disclosed in seven pending utility patent applications in the U.S., one European granted patent validated in eight countries, two issued foreign patents and 23 pending foreign patent applications that relate to the company's GoCAR-T technology. If U.S. patents issue from the U.S. applications, the estimated expiration date of the last to expire patent is in 2039. If patents are issued in foreign jurisdictions, the anticipated expiration dates will be in 2039.
Pursuant to the company's licenses from Baylor College of Medicine and Ariad Pharmaceuticals, Inc., the company has exclusive commercial rights to 12 issued U.S. patents expiring in 2024 or later, four pending U.S. utility patent applications, three European granted patents (the first validated in three countries, the second validated in nine countries, and the third validated in four countries), five issued foreign patents expiring in 2024 or later and two pending patent applications in foreign jurisdictions that relate to the company's GoCAR-T, rivo-cel and certain of the company's other technologies. If U.S. patents issue from the pending U.S. patent applications, the estimated expiration date of the last to expire patent is 2031. If patents from the pending patent applications are issued in foreign jurisdictions, the estimated expiration dates range from 2024 to 2029.
Pursuant to the company's license agreement with Agensys, Inc. the company has exclusive commercial rights for technology to target certain cancer-specific antigens.
Collaboration and License Agreements
Co-Development and Co-Commercialization Agreement - Adaptimmune
In December 2016, the company and Adaptimmune Therapeutics plc, or Adaptimmune, entered into a Co-Development and Co-Commercialization Agreement, or the Adaptimmune Agreement, in order to facilitate a staged collaboration to evaluate, develop and commercialize next generation T cell therapies. Since the company has stopped all development activity related to its product candidates, the company is not performing any development efforts under this agreement.
Under the Adaptimmune Agreement, the parties agreed to evaluate the company's GoTCR technology, iMC co-stimulation, with Adaptimmune's affinity-optimized SPEAR T cells for the potential to create enhanced TCR product candidates.
License Agreement - Agensys
In December 2015, the company and Agensys, Inc., or Agensys, entered into a license agreement, or the Agensys Agreement, pursuant to which Agensys granted the company, within the field of cell and gene therapy of diseases in humans, an exclusive, worldwide license and sublicense to its patent rights directed to PSCA and related antibodies; and the company granted Agensys a non-exclusive, fully paid license to the company's patents directed to inventions that were made by the company in the course of developing its licensed products, solely for use with Agensys therapeutic products containing a soluble antibody that binds to PSCA or, to the extent not based upon the company's other proprietary technology, to non-therapeutic applications of antibodies not used within the field.
License Agreement - BioVec
In June 2015, the company and BioVec Pharma, Inc., or BioVec, entered into a license agreement, or the BioVec Agreement, pursuant to which BioVec agreed to supply the company with certain proprietary cell lines and granted the company a non-exclusive, worldwide license to certain of its patent rights and related know-how related to such proprietary cell lines.
License Agreements - Baylor College of Medicine
2008 Baylor License Agreement
Pursuant to an Exclusive License Agreement with Baylor College of Medicine, or Baylor, dated March 20, 2008, or the 2008 Baylor license agreement, the company obtained an exclusive, worldwide and fully paid up license to certain intellectual property, including intellectual property related to methods for activating antigen presenting cells and to genetic constructs coding for membrane bound inducible cytoplasmic CD40.
2010 Baylor License Agreement
Pursuant to an Exclusive License Agreement with Baylor, dated June 27, 2010, or the 2010 Baylor license agreement, the company obtained an exclusive, worldwide license to certain intellectual property, including intellectual property related to methods for treating prostate cancer, methods of administering T cells to a patient, and methods of activating antigen presenting cells with constructs comprising MyD88 and CD40.
2014 Baylor License Agreement
Pursuant to an Exclusive License Agreement with Baylor, effective November 1, 2014, or the 2014 Baylor license agreement, the company obtained an exclusive, worldwide license to certain intellectual property, including intellectual property related to methods for inducing selective apoptosis.
Government Regulation and Product Approval
Biologics, such as the company's engineered cells are regulated through the FDA's Center for Biologics Evaluation and Research, or CBER, while synthetic drugs are regulated through the FDA's Center for Drug Evaluation and Research. The company's product candidates must be approved by the FDA before they may be legally marketed in the U.S. and by the appropriate foreign regulatory agency before they may be legally marketed in foreign countries.
In the U.S., the company's activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including but not limited to, the Centers for Medicare and Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services, or HHS, such as the Office of Inspector General, the U.S. Department of Justice, or DOJ, and individual U.S. Attorney offices within the DOJ, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the false claims laws, the privacy provisions of the Health Insurance Portability and Accountability Act, or HIPAA, the sunshine provisions of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the PPACA and similar state laws, each as amended.
The Foreign Corrupt Practices Act obligates companies whose securities are listed in the U.S. to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
In addition to the foregoing, state and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservancy and Recovery Act and the Toxic Substances Control Act, affect the company's business.
History
Bellicum Pharmaceuticals, Inc. was founded in 2004. The company was incorporated in Delaware in 2004.
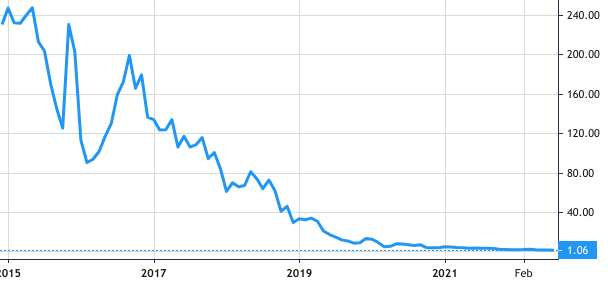
 Stock Value
Stock Value

