About Agile Therapeutics
Agile Therapeutics, Inc. operates as a women’s healthcare company. The company is dedicated to fulfilling the unmet health needs of women. The company is committed to innovating in women’s healthcare where there continues to be unmet needs.
The company’s first and only product, Twirla, is a once-weekly prescription combination hormonal contraceptive patch. It exposes patients to an estrogen dose consistent with commonly prescribed combined hormonal contraceptives, or CHCs, and is lower than the estrogen dose found in other marketed contraceptive patches. Twirla leverages the company’s proprietary transdermal patch technology called Skinfusion. Skinfusion is designed to allow drug delivery through the skin while promoting patch adhesion and patient comfort and wearability, which may help support compliance.
The company focuses on its advancement as a commercial company. The company can grow Twirla by leveraging its partnerships in the retail and non-retail channels. For example, the company initiated a relationship with Nurx, which can make Twirla nationally available on Nurx.com, part of the Thirty Madison telehealth platform focused on sexual and reproductive health. Nurx has provided contraception to over 1 million patients. In addition to growing Twirla, the company plans to continue pursuing opportunities to broaden its portfolio to address areas of unmet medical need in women’s health.
Strategy
The company’s resources are focused on the commercialization of Twirla. The company also expects to continue exploring possible expansion through business development activities, such as acquiring access to new products through in-licensing, co-promotion or other collaborative arrangements.
The company’s priorities are to continue to manage its available cash and obtain financing to fund its business plan without delay; continue to implement its commercialization plans for Twirla to increase uptake of Twirla in the United States, through targeted digital direct to consumer advertising, growing its telemedicine presence through new partnerships and its existing partnership with Nurx, and driving growth in the non-retail channel through its collaboration with Afaxys, which provides it access to some of the largest Planned Parenthood organizations in the country; continue to expand access to Twirla through multiple business channels, including retail and specialty pharmacies, telemedicine, government contracting, and non-retail channels, including public health centers through its relationship with Afaxys; expand coverage and reimbursement for Twirla in the United States from private and public third-party payors; maintain and manage the supply chain for Twirla to support increased commercialization of Twirla across the United States and working through existing and future inventory prior to product becoming short-dated; evaluate the advancement of its existing pipeline and its possible expansion through business development activities; and continue to implement its obligations related to its post-marketing commitment and the post-marketing requirement studies of Twirla.

Twirla
Twirla is the company’s first and only approved product. Twirla received FDA approval on February 14, 2020, as a method of contraception for use in women of reproductive potential with a body mass index (BMI) < 30 kg/m2 for whom a combined hormonal contraceptive is appropriate. Based on the reduced efficacy seen with increasing BMI in a Phase 3 clinical trial, Twirla’s limitation of use instructs healthcare providers to consider Twirla’s reduced effectiveness in women with a BMI = 25 to <30 kg/m2 before prescribing. Twirla is contraindicated in women with a BMI = 30 kg/m2 because, compared to women with a lower BMI, women in this group had reduced effectiveness and may have a higher risk for VTEs. Twirla’s label also includes the class-wide boxed warning, contraindications, and warnings and precautions applicable to all combined hormonal contraceptives, or CHCs.
Twirla is a prescription combined hormonal contraceptive patch that contains the active ingredients ethinyl estradiol, or EE, which is a synthetic estrogen, and levonorgestrel, or LNG, which is a type of progestin, both of which have an established history of efficacy and safety in marketed combination oral contraceptives. Twirla delivers 30 micrograms of EE per day, a dose of EE consistent with the dose delivered by many commonly prescribed oral contraceptives. Twirla is the contraceptive patch that contains LNG, a widely prescribed progestin. The company’s Skinfusion technology allows Twirla to be the first approved patch capable of delivering a contraceptive dose of LNG across the skin. The patch is applied once weekly for three weeks, followed by a week without a patch. Twirla is packaged with three individually wrapped patches per carton to provide for one 28-day cycle of therapy.
Twirla’s approval is primarily based on safety and efficacy data from the Phase 3 SECURE trial. The SECURE trial was a new approach to clinical trials, and was intentionally designed to include broad enrollment criteria and a patient population of women likely to use hormonal contraceptives.
The SECURE trial was a multi-center, single-arm, open-label, 13-cycle trial that evaluated the safety, efficacy and tolerability of Twirla in 2,031 healthy women, aged 18 and over, at 102 experienced investigative sites across the United States. The trial was designed in consultation with the FDA, and incorporated a number of stringent trial design elements, including exclusion of treatment cycles not only for use of backup contraception but also for lack of sexual activity. SECURE had broad entry criteria, placed no limitations on body mass index, or BMI, or other demographic factors during enrollment, and enrolled a large and diverse population from the United States in order to allow for efficacy to be assessed across different groups. These entry criteria resulted in the inclusion of a substantial number of women with high BMIs, who have frequently been underrepresented in prior contraceptive studies. The efficacy measure for SECURE was the Pearl Index in an intent-to-treat population of subjects 35 years of age and under. The FDA also requested the inclusion of prespecified efficacy analyses related to BMI and body weight.
As part of Twirla’s approval, and consistent with requirements for another approved CHC, the FDA is requiring the company to conduct a long-term prospective, observational post-marketing study, or PMR, comparing the risks for VTE and ATE in new users of Twirla to new users of CHCs. In January 2023, the FDA agreed with its proposal to address this PMR using electronic health records (EHR) and insurance claims from a large database from multiple healthcare systems. The FDA also agreed to extend the study timelines. Under these new milestones, interim safety data reporting to the FDA is due in November 2029, and the final PMR study report is scheduled to be submitted to the FDA in November 2035. As part of Twirla’s approval, the company also agreed to an FDA-requested post-marketing commitment, or PMC, study to assess the residual drug content and strength of Twirla in a minimum of 25 women. The PMC study is similar to residual drug studies requested of patch developers in the FDA’s November 2019 draft guidance entitled Transdermal and Topical Delivery Systems—Product Development and Quality Considerations. The PMC study was completed in the fourth quarter of 2021, and the study report was submitted to the FDA in June 2022. The company continues to discuss the results with the FDA.
Strategic Agreements
Agreement with Corium
In April 2020, the company entered into a Manufacturing and Commercialization agreement with Corium Pharma Solutions (Corium), which it refers to as the Corium Agreement, and which replaced its previous development agreement. Corium continues to operate under the Corium Agreement after the Corium Reorganization. Pursuant to the Corium Agreement, Corium will manufacture and supply all of the company’s product requirements for Twirla at certain specified rates. Under the terms of the Corium Agreement, Corium is to be the exclusive supplier of Twirla for ten years.
Agreement with Syneos Selling Solutions
In April 2020, the company entered into a project agreement with inVentiv Commercial Services, LLC, or inVentiv, a Syneos Health Group Company, which it refers to as the Syneos Agreement, under its Master Services Agreement with inVentiv. Pursuant to the Syneos Agreement, inVentiv, through its affiliate Syneos Selling Solutions, will provide a field force of sales representatives to provide certain detailing services, sales operation services, compliance services and training services with respect to Twirla to the company in exchange for an up-front implementation fee and a fixed monthly fee. Effective February 1, 2022, the company entered into an amendment to the Syneos Agreement that extended the term until August 23, 2024. At that time, the Syneos Agreement will terminate automatically unless extended upon the mutual written agreement of the parties.
Research and Development
For the year ended December 31, 2022, the company’s research and development expenses included $3.3 million.
Intellectual Property
More specifically, Twirla is a transdermal contraceptive hormone delivery system. The system is a patch for application to the skin and contains two API, the hormones LNG, which is a synthetic progestin, and EE, a synthetic estrogen.
In the company’s Twirla product candidate line the active adhesive system consists of the active ingredients in a polyacrylate adhesive polymer matrix comprising the permeation enhancers dimethylsulfoxide, ethyl lactate, capric acid and lauryl lactate. The active blend is coated onto a release liner, and a backing layer is added on top of the active blend. The peripheral adhesive system comprising three layers, also called the overlay, is added onto the backing layer. The overlay comprises a polyisobutylene adhesive layer, an acrylic adhesive layer, and an overlay covering. The overlay covering is a commercially available silk-like polyester fabric. The adhesive components of the overlay, in addition to their adhesive function, create an in situ seal with the disposable release liner, trapping evaporable solvents in the active blend, thereby extending the usable shelf life of the product candidate and contributing to the comfort and effectiveness of the transdermal system during use.
Three U.S. patents are listed in the FDA’s Orange Book. Five other previously-listed U.S. patents have expired. Of those expired U.S. Patents, foreign counterparts have been granted and remain in force in China, Hong Kong, India, Israel and Mexico. Those patents are directed to the dried final product formulation used in Twirla and to methods of administration.
U.S. Patent Nos. 8,246,978, 8,747,888, and 9,050,348, currently listed in the Orange Book, are directed to structural features of the transdermal delivery system used in Twirla patch design for transdermal delivery of hormones or of other drugs. As such, these patents protect a platform technology for delivery of LNG, EE, other hormones, and other drugs. These patents expire in July and August 2028. Foreign counterparts have been granted in Australia, Brazil, Canada, Eurasia, Switzerland, Germany, Spain, France, the United Kingdom, Hong Kong, Ireland, India, Italy, Japan, the Netherlands, New Zealand and Japan.
U.S. Patent Nos. 9,198,876, 9,192,614, 9,198,919, 9,198,920, 9,775,847 and 9,782,419 and related patents and patent applications are directed to various novel dosing regimens, each of which employs transdermal delivery of contraceptive doses of EE and LNG during a treatment interval and transdermal delivery of low dose EE and low dose LNG during a withdrawal interval. Foreign counterparts are granted in Europe and Canada. The company expects these patents will be relevant to two of the products in its pipeline, AG200-SP and AG200-ER, as well as other new potential regimens. These patents expire in October 2029.
U.S. Patent No. 9,364,487 is directed to a composition and device for transdermal delivery of LNG for P-only therapy. The composition contains an anti-oxidant to protect the progestin against oxidative degradation caused by other components of the composition. Foreign counterparts are granted in Canada, Europe, Hong Kong, India, Japan and Mexico. Though not relevant to any current pipeline products, these patents may be useful for protection of future products. These patents expire in November 2032.
The company has patent applications pending in the United States and certain foreign jurisdictions directed to novel formulations and methods designed to improve efficacy and modulate side effects of administration, as well as to provide personalized dosing based on body weight or BMI.
Regulatory Exclusivity
The company’s NDA for Twirla was submitted under Section 505(b)(2) of the FDCA. Even though Twirla utilizes API that were previously approved in the United States, Twirla utilizes LNG in a new dosage form, specifically a transdermal patch, and the company provided new clinical data essential to approval in its NDA to establish the safety and efficacy of Twirla. Therefore, the company received three years of U.S. marketing exclusivity for Twirla under the Hatch Waxman Act.
History
Agile Therapeutics, Inc. was founded in 1997. The company was incorporated under the laws of the state of Delaware in 1997.

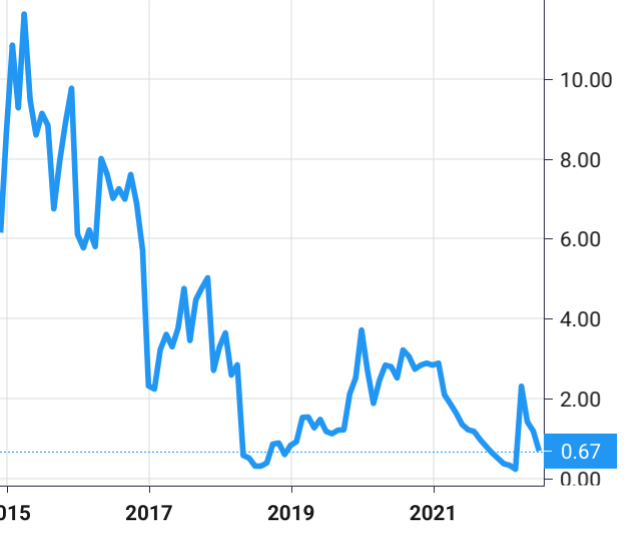
 Stock Value
Stock Value

