About Asensus Surgical
Asensus Surgical, Inc. operates as a medical device company.
The company is digitizing the interface between the surgeon and patient to reimagine surgery and pioneer a new era of surgery that the company refers to as, Performance-Guided Surgery (PGS), by unlocking clinical intelligence to enable surgeons to deliver consistently superior outcomes to patients and establish a new standard of surgery.
Built upon the foundation of laparoscopic minimally invasive surgery, that remains the gold standard of surgery today, with the Senhance Surgical System, combined with the Intelligent Surgical Unit (ISU), the company is pioneering this new standard of surgery, PGS, to increase surgeon control and reduce surgical variability. With the addition of machine vision, Augmented Intelligence, and deep learning capabilities throughout the surgical experience delivered via the ISU, the company intends to holistically address the current clinical, surgeon performance (fatigue and ergonomics), and the economic shortcomings that drive surgical outcomes in a value-based healthcare environment.
On February 21, 2023, the company held an investor day to describe the company’s focus on developing a next generation robotic system the company calls the LUNA Surgical System and the ongoing developments in the company’s Performance-Guided Surgery platform.
Performance-Guided Surgery is consisted of three strategic pillars: enhanced robotic precision and manipulation capabilities, via the Senhance System today and, when developed and approved, the LUNA System; expanded intra-operative Augmented Intelligence clinical decision support guidance for the surgeon via the ISU; and the integration of cloud and big data to harness best practices across pre-, intra- and post-operative settings, and make it available to surgeons around the world via the Asensus Cloud.
In the LUNA System, the company is developing a ‘best in class’ robot that will use 3mm and 5mm instruments (as contrasted with current systems available that use 8mm instruments), TrueWrist fully wristed 5mm instruments, monopolar and bipolar electrosurgery capabilities, rapid instrument exchange with the company’s proprietary instrument drive system, an open platform with a smaller footprint in the OR, as compared to the Senhance System, up to four-arm configuration with enhanced manipulation and dexterity, a surgeon console with 4K-3D capabilities without the need to wear 3D glasses, and unconstrained handles with improved digital features while retaining haptic feedback.

The LUNA System will continue the company’s tradition of providing instruments that are reusable and can be re-sterilized and re-processed, and, with improvements in manufacturing, are expected to have lower costs per procedure.
The company also clarified its focus areas for the company’s future development efforts with the ISU, including:
An analytical feature set, which includes intra-operative surgical planning that will allow surgeons to map out and plan for specific surgical actions intraoperatively using the ISU’s Augmented Intelligence;
A safety feature set, which includes real-time notifications that will enable the identification and marking of potential hazards during the operation, and optionally restrict access to these structures or areas by simultaneously controlling the robotic arm; and
A training and education set, allowing multiple team members to work together in real-time by annotating, highlighting and drawing on a shared visual display of the surgical field to communicate and provide expert support in real-time.
The Asensus Cloud is being designed to assist in pre-operative surgical planning, post-surgical performance analytics and best practices guidance, and enable the extraction and aggregation of insights from surgical data. The collection and analysis of surgical data transformed into insights, and when shared with the company’s physicians, will enhance surgical planning, surgeon education and training, and promote better patient outcomes.
The company anticipates that, although the company has been working on aspects of the LUNA System for four years, the company will spend the rest of 2023 refining and finalizing the design builds, and conducting testing on the ISU enhancements for standalone use. The company anticipates reaching design freeze on the LUNA System in the first quarter of 2024 and making regulatory notices and submissions by the end of 2024. For the ISU enhancements and standalone configuration, the company anticipates making regulatory notices and submissions in the first quarter of 2024 and beginning commercial activities by mid-2024. The company anticipates a commercial pilot launch of the LUNA System in the second half of 2025.
The company is focusing on markets with high utilization of laparoscopic techniques, including Japan, Western Europe and the United States. The company’s focus in 2022 was on (1) increasing the number of placements of the Senhance System, not necessarily through sales, but through leasing arrangements, (2) increasing the number of procedures conducted using the Senhance System quarter over quarter, and (3) solidifying key opinion leader support and publications related to the value of the Senhance System in laparoscopic procedures. The company is not focusing on revenue targets, especially in the United States.
The company has six global training sites, including three in the United States at the Advent Health Nicholson Center in Celebration, Florida, at LSU Health Sciences Center, New Orleans, and the company’s office in Research Triangle Park North Carolina; two in Europe at Amsterdam Skills Centre, and the company’s office in Milan, Italy; and one in Asia at the company’s office in Tokyo, Japan.
Product
The company is addressing the challenges in laparoscopy and robotic-assisted surgery with technologically advanced products and product candidates that leverage the best features of both approaches to minimally invasive surgery, or MIS. The company is also addressing the need for clinically relevant data and analysis through the company’s PGS offerings.
The Senhance Surgical System
The Senhance System addresses key challenges for laparoscopic surgeons and hospitals by delivering the benefits of robotics with improved control of the surgical field, enhanced visualization and camera control and improved ergonomics, coupled with the familiarity of laparoscopic motion and consistent per-procedure costs.
The Senhance System is available for sale in Europe, the United States, Japan, Taiwan, the Commonwealth of Independent States, or CIS, and select other countries.
The Senhance System has a CE Mark in Europe for adult and pediatric laparoscopic abdominal and pelvic surgery, as well as limited thoracic surgeries excluding cardiac and vascular surgery, and surgeries in direct contact with central nervous systems.
In the United States, the company has 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use of the Senhance System in general laparoscopic surgical procedures and laparoscopic gynecologic surgery in a total of 31 indicated procedures, including benign and oncologic procedures, laparoscopic inguinal, hiatal and paraesophageal hernia, sleeve gastrectomy and laparoscopic cholecystectomy (gallbladder removal) surgery.
In Japan, the company has received regulatory approval and reimbursement for 124 laparoscopic procedures.
The Senhance System received its registration certificate by the Russian medical device regulatory agency, Roszdravnadzor, allowing for its sale and utilization throughout the Russian Federation.
The company also enters into lease arrangements with certain qualified customers. For some lease arrangements, the customers are provided with the right to purchase the leased Senhance System during or at the end of the lease term (which the company refers to as a Lease Buyout).
The company’s focus is on seeking regulatory approvals and clearances for the Senhance System and related product offerings and instruments and pursuing commercialization of the company’s products.
On July 28, 2021, the company received FDA clearance for 5mm diameter articulating instruments, offering better access to difficult-to-reach areas of the anatomy by providing two additional degrees of freedom. These instruments previously received CE Mark for use in the EU.
The company also focused on expanding the indications for use of the Senhance System. As of March 2021, the Senhance System is FDA cleared for use in general laparoscopic surgical procedures and laparoscopic gynecologic surgery, including a total of 31 indicated procedures, including benign and oncologic procedures, laparoscopic inguinal, hiatal and paraesophageal hernia, sleeve gastrectomy and laparoscopic cholecystectomy. The company continues to make additional submissions for clearance or approval for enhancements to the Senhance System and related instruments and accessories, including additional filings and approvals sought in Japan.
The Senhance System is a multi-port robotic surgery system that allows up to four arms to control robotic instruments and a camera. The system builds on the success of laparoscopy by enhancing the traditional features that surgeons have come to expect from existing products and by addressing some of the limitations associated with robotic surgery systems for laparoscopic procedures. The Senhance System also offers responsible economics to hospitals through its robotic technology coupled with reusable standard instruments that yield minimal additional costs per surgery when compared to laparoscopy. The Senhance System has a CE Mark in Europe for laparoscopic abdominal and pelvic surgery, as well as limited thoracic operations excluding cardiac and vascular surgery, and surgeries in direct contact with central nervous systems.
The Senhance System is manufactured for the company by third-party contract manufacturers. The company or its manufacturers acquire raw materials and components of the Senhance System from vendors, some of which are sole suppliers. The company’s relationships with its vendors and manufacturing contractors are good. The company has the manufacturing capacity and inventory reserves to meet the company’s anticipated Senhance System sales for the foreseeable future.
ISU and Digital Solutions
The company’s ISU is a real-time intra-operative surgical image analytics platform that leverages Augmented Intelligence to help reduce surgical variability and provides tools to reduce a surgeon’s cognitive fatigue. It is used to enable machine vision capabilities on the Senhance System and collect clinical data related to surgical procedures. The ISU was developed using image analytics technology that the company acquired as part of the company’s October 2018 acquisition of the assets, intellectual property and highly experienced multidisciplinary personnel of Medical Surgical Technologies, Inc., or MST, an Israeli-based medical technology company. On March 13, 2020, the company had received FDA clearance for the ISU. On September 23, 2020, the company announced the first surgical procedures successfully completed using the ISU. On January 19, 2021, the company received CE Mark for the ISU, allowing the company to expand its Augmented Intelligence capabilities to all global areas accepting the CE Mark.
The ISU enables machine vision-driven control of the camera for a surgeon by responding to commands and recognizing certain objects and locations in the surgical fields and allows a surgeon to change the visualized field of view using the movement of their instruments. In September 2021 the company received FDA clearance for additional Augmented Intelligence features of the ISU, and received CE Mark for such additional features in January 2023. The newest ISU features expand upon these capabilities and introduce additional advanced features, including 3D measurement, digital tagging, image enhancement, and enhanced camera control based on real-time data while performing surgery. The regulatory review of such expanded capabilities, which included a review of the Senhance System platform, made Senhance one of the first robotic surgical systems to be approved through the new, more rigorous EU Medical Device Regulation, or MDR, process.
The company is continuing to advance the utility of the ISU for the Senhance System while also adding such capabilities to the standalone ISU and the LUNA System.
The company’s digital solutions are the features, products and platforms that are enabled by data, generated through the digitization of the interface between the surgeon and the patient, and deployed via software. The company’s digital solutions are a foundational component of the company’s PGS strategy enabling the delivery of insights in pre-operative and post-operative settings, while continuously enhancing the company’s real-time, intra-operative Augmented Intelligence offerings. Given the criticality of this part of the company’s business, in 2022 the company reorganized the company’s R&D structure to establish a dedicated engineering team focused on digital solutions and established an additional cross-functional team to advance efforts in data collection, connectivity, needs definition and solution development. Commercially available digital solutions are largely deployed via the ISU in the form of Augmented Intelligence applications including automatic camera control, digital tagging, and digital measurement. To develop these and future digital solutions, the company designed and deployed the Asensus Cloud, which has been architected to efficiently manage unique surgical data automatically transferred via connected ISUs and additional sources. The Asensus Cloud provides a secure, scalable, and efficient platform for data storage, data use and computing in machine learning model development, business model innovation and future analytics delivery via portals and dashboards. These analytics solutions will address numerous challenges in the pre-operative planning and post-operative assessment phases of surgery, enhancing training, as well as continuing education, to help advance Performance-Guided Surgery and promote consistently superior outcomes.
Senhance Connect
Senhance Connect is a telesurgical platform that allows surgeons in an operating room to connect with and communicate with other Senhance surgeons in other locations. The process allows for streaming of multiple operating room camera views and an endoscopic view simultaneously, and allows for two-way screen sharing and annotation. This product is part of the company’s PGS, enabling the ability to provide real-time digital collaboration capabilities to surgeons enabling best practice sharing and surgical proctoring to a wider audience. Additionally, this expands surgeon flexibility and is more cost effective, enabling broader global access to clinical excellence.
Clinical Registry (TRUST)
TRUST is the largest multi-specialty robotic-assisted laparoscopic registry in the industry. In 2022, the company continued to leverage the growing body of real-world clinical data through the utilization of the company’s TRUST clinical registry, which is aimed at providing a research tool that enables physicians to monitor safety, efficacy, and feasibility of robotic assisted surgical interventions in a variety of abdominal, thoracic, urologic and gynecologic surgical cases using the Senhance System. In 2022, the company continued to drive enrollment, as well as support peer-reviewed publications through this registry.
Instruments and Other Products
Instruments
The company successfully obtained FDA clearance and CE Mark for a number of instruments, including the company’s 3mm diameter instruments, the company’s 3mm and 5mm hooks, and articulating instruments. The 3mm instruments enable the Senhance System to be used for microlaparoscopic surgeries, allowing for tiny incisions. The company offers approximately 80 instruments and accessories in the company’s portfolio. The company also has designed the Senhance System so that third-party manufactured instruments can be easily adapted for use.
The company’s articulating instruments were commercially launched in the U.S. and Japan in the fourth quarter of 2022.
Other Products
The Senhance ultrasonic system is an advanced energy device used to deliver controlled energy to ligate and divide tissue, while minimizing thermal injury to surrounding structures.
Indications for Use
The company continues to work on expanding the indications for use of the Senhance System and the company’s instruments and other products. The most notable recent advances are:
The company received CE Mark approval for an expanded indication to treat pediatric patients.
In 2020, the company submitted a notice to the FDA for 510(k) clearance for expanded General Surgery indications for use for the Senhance System. In March 2021, the company received such clearance which allows for hiatal and paraesophageal hernia, and sleeve gastrectomy procedures. These additional indications helped to increase procedure volume in 2022.
The company submitted a 510(k) notice to the FDA for expanding the indications for use of the Senhance System to pediatric patients.
Business Strategy
The company’s strategy is to focus on the realization of Performance-Guided Surgery through the continued collection of surgical data via the ISU and Asensus Cloud leveraging the Senhance System and by other means of non-robotic laparoscopic surgery, while completing the design and development of the LUNA System and its capabilities.
The company continues the market development for and commercialization of the Senhance Surgical System, which digitizes laparoscopic minimally invasive surgery, or MIS. The Senhance System is the first and only multi-port, digital laparoscopy platform designed to maintain laparoscopic MIS standards while providing digital benefits, such as haptic feedback, robotic precision, improved ergonomics, advanced instrumentation, including 3mm microlaparoscopic instruments, 5mm articulating instruments, eye-sensing camera control and fully-reusable standard instruments to help maintain per-procedure costs similar to traditional laparoscopy.
The company’s strategy is to focus its resources on the market development of digital surgery to create a new and unique market segment for Performance-Guided Surgery using the standalone ISU and the LUNA System in the future and the Senhance System, the ISU and offered instruments to generate procedural data to inform and elevate practice in real time.
Sales and Marketing
The company utilizes distributors in jurisdictions where the company does not sell directly. The company’s distribution agreements typically provide exclusivity in a specific territory or jurisdiction.
The company is dependent on growing the number of hospital customers and increasing the number of customers with installed Senhance Systems. Throughout 2022, the company initiated nine Senhance surgical programs, four in Germany, three in Japan and two in the CIS region. The company defines the initiation of a Senhance Surgical program as entering into an agreement to purchase or lease, and subsequently utilizing a Senhance System.
Intellectual Property
The following summarizes the company’s patent and patent application portfolio.
As of December 31, 2022, the company’s patent portfolio included approximately 75 issued or allowed United States patents, over 100 patents issued outside the United States, and more than 130 patent applications filed in the United States and internationally. The company owns all right, title and interest in all but the approximately 38 of the company’s patents and patent applications that are exclusively licensed to the company and the approximately 25 patents and patent applications that are non-exclusively licensed to the company.
Several of the company’s issued patents resulted from filings related to the Senhance System. These include 8 United States patents, and approximately 40 patents outside the United States. The earliest to expire U.S. and non-U.S. patents within this part of the company’s portfolio will remain in force until 2027. The patent applications include over 120 that relate to the Senhance System, the LUNA System, the ISU or other features, instruments, or components for robotic-assisted surgery. The company’s patents and applications that the company acquired from MST relate to image analytics, the company’s digital solutions and robotic surgery, among other things. The company intends to continue to seek further patent and other intellectual property protection in the United States and internationally, where available and when appropriate, as the company continues its product development efforts.
Some of the company’s issued patents and pending applications for the Senhance System, as well as associated technology and know-how, are exclusively licensed to Asensus Surgical Italia from the European Union. The license agreement with the European Union has a term, which runs until the final licensed patent expires unless the agreement is terminated earlier by mutual consent of the parties, for the company’s convenience, or for breach. The company is in compliance with the terms of this license agreement.
Government Regulation
The company’s products are subject to premarket notification and clearance under section 510(k) of the FDCA, and the 510(k) process is normally used for products of the type that the company is developing and propose to market and sell. To obtain 510(k) clearance, the company must submit to the FDA a premarket notification (510(k) notice) demonstrating that the proposed device is ‘substantially equivalent’ to a predicate device already on the market.
The Senhance System and many related products are Class II devices as evidenced by the company’s cleared 510(k) notices.
As manufacturers, the company and its suppliers are subject to announced and unannounced inspections by the FDA to determine the company’s compliance with the Quality System Regulation, or QSR (21 CFR Part 820), and other regulations.
In Europe, the company complies with the requirements of the 93/42/EEC Medical Devices Directive, or MDD, and appropriately affix the CE Mark on the company’s products to attest to such compliance. As legal manufacturers, the company and its suppliers are subject to announced and unannounced inspections by the European Notified Bodies and Competent Authorities.
The company’s business activities are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which the company conducts its business. These laws include the following:
The U.S. Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving anything of value to induce (or in return for) the referral of business, including the purchase of a particular medical device reimbursable under Medicare, Medicaid or other federally financed healthcare programs.
The federal civil False Claims Act, or FCA, which prohibits, among other things, any person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of federal funds, or knowingly making, or causing to be made, a false statement material to a false claim.
The Health Insurance Portability and Accountability Act of 1996 and its implementing regulations (collectively ‘HIPAA’) prohibits, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors.
The majority of states also have statutes or regulations similar to the federal anti-kickback and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
The U.S. Physician Payment Sunshine Act, or Sunshine Act, requires tracking of payments and transfers of value to physicians and teaching hospitals and ownership interests held by physicians and their families, and reporting to the federal government and public disclosure of these data.
The company has market development and commercial activities in a number of international markets and intends to focus on such markets in the near term. Some of these markets maintain unique regulatory requirements outside of or in addition to those of the FDA and the European Union. The Senhance System is CE Marked, which is the basis to allow the company to offer the product for sale in a number of jurisdictions, including select countries in Europe, the Middle East and Asia.
Research and Development
The company’s research and development expenses were $28.9 million for the year ended December 31, 2022.
Competition
The company’s surgical competitors include, but are not limited to: Medtronic plc, Intuitive Surgical Inc., Vicarious Surgical, Inc., Momentis Surgical, Distalmotion SA, and CMR Surgical Ltd., as well as Activ Surgical, Inc., Theator Surgical, and CareSyntax Inc.
History
The company was founded in 2006. It was formerly known as TransEnterix, Inc. and changed its name to Asensus Surgical, Inc. in 2021.

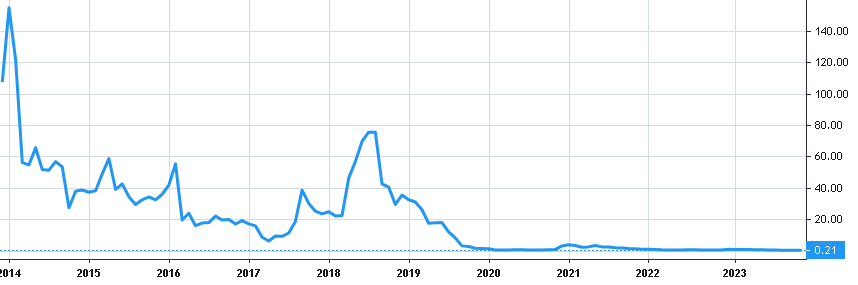
 Stock Value
Stock Value

