About Taro Pharmaceutical Industries
Taro Pharmaceutical Industries Ltd. (Taro Israel) operates as a multinational, science-based pharmaceutical company. The company is a subsidiary of Alkaloida Chemical Company Exclusive Group Ltd.
The company develops, manufactures, and markets Rx and OTC pharmaceutical products primarily in the U.S., Canada, Israel, and Japan. The company’s primary focus includes semi-solids formulations, such as creams and ointments and other dosage forms, such as liquids, capsules, and tablets, in the dermatological and topical, cardiovascular, neuropsychiatric, and anti-inflammatory therapeutic categories.
The company operates principally through Taro Israel and its following subsidiaries (including indirect): Taro Pharmaceuticals Inc. (Taro Canada), Taro U.S.A., and those entities relating to the Alchemee business, including Alchemee LLC, The Proactiv Company KK, and Alchemee Skincare Corporation (collectively, Alchemee). The principal activities and primary product lines of Taro Israel and these subsidiaries may be summarized as follows:
Taro Israel: Taro Israel manufactures more than 100 finished dosage form pharmaceutical products for sale in Israel and for export. Taro Israel produces APIs used in the manufacture of finished dosage form pharmaceutical products. Taro Israel markets and distributes both proprietary and generic products in the local Israeli market. Taro Israel performs research and development.
Taro Israel’s primary product lines include dermatology: Rx and OTC semi-solid (creams, ointments, lotions, foams and gels) and liquid products; cardiology and neurology: prescription oral dosage products; analgesics, Rx and OTC oral dosage products; central nervous system (CNS) – Rx oral dosage products; and allergy (Antihistamine): OTC oral dosage products.
Taro Canada: Taro Canada manufactures more than 200 finished dosage form pharmaceutical products for sale in Canada and for export to the U.S. and other markets. Taro Canada markets and distributes both proprietary and generic products in the Canadian market. Taro Canada performs research and development.

Taro Canada’s primary product lines include dermatology: Rx and OTC semi-solid products (creams, ointments, lotions and gels) and liquid products; and allergy (Antihistamine): OTC oral dosage products.
Taro U.S.A.: Taro U.S.A. markets and distributes both proprietary and generic products in the U.S. market. Taro U.S.A performs regulatory, post marketing and clinical activities.
Taro U.S.A.’s primary product lines include dermatology: Rx and OTC semi-solid products (creams, ointments, lotions, foams and gels) and liquid products; cardiology and Neurology: Rx oral dosage products; and other Rx and OTC products.
Alchemee: Alchemee markets and distributes dermatologic products in the U.S., Canada, Japan and other markets. Alchemee’s primary product lines dermatology products (creams, ointments, lotions, and solutions) Proactiv solution, Proactiv+, and ProactivMD.
As of March 31, 2023, 19 (excluding tentative approvals) of the company’s ANDAs are being reviewed by the FDA. During the fiscal year ended March 31, 2023, the company filed 7 ANDAs with the FDA. In addition, there are numerous products for which either development or internal regulatory work is in process. The applications pending before the FDA are at various stages in the review process, and there can be no assurance that it will be able to successfully complete any remaining testing or that, upon completion of such testing, approvals will be granted.
On February 28, 2022, Taro U.S.A. acquired the Alchemee business from Galderma. The acquisition includes Alchemee’s business and assets worldwide, including the Proactiv brand. The acquisition expands the company’s product portfolio in OTC dermatology products. Taro U.S.A. assigned its entire ownership of the Alchemee business to the company.
Products
The company markets more than 200 pharmaceutical products in over 24 countries. The following represents key therapeutic categories and dosage forms.
Therapeutic Categories
The following represents various key therapeutic categories: allergy, analgesic, antibacterial, antibiotic, anticonvulsant, antiemetic, antifungal, anti-inflammatory, anti-cancer, antiplatelet agent, antipyretic, cardiovascular, CNS, corticosteroid, cosmetic, cough and cold, dermatology, diuretic, endocrine, gastrointestinal, laxative, narcotics, neuropathic pain, neuropsychiatric, sedative/hypnotic, and topical anti neoplastic.
Dosage Forms
The following represents various dosage forms of products: capsule, cream, drops, emulsion, gel/gel kit, granules, injectable, lotion, oil, ointment, paste (including dental), powder/powder for solution, rectal suppository, shampoo, solution/solution for infusion, spray, suspension, syrup, tablets, toothpaste and mouthwash, topical foam, and topical solution.
Topical corticosteroids are used in the treatment of some dermatologic conditions (including psoriasis, eczema, and various types of skin rashes). Topical antineoplastics are used in the treatment of cancer (including skin cancer). Antifungals are used in the treatment of some infections (including athlete’s foot, ringworm and vaginal yeast infections). Anticonvulsants are used in the treatment of various seizure disorders (including epilepsy). Cardiovascular products are used in the treatment of heart disease. There are several categories of cardiovascular drugs, including anticoagulants, antihypertensive, and antiarrhythmic. Anticoagulants, commonly known as blood thinners, are used in the treatment of heart disease and stroke associated with heart disease. Some of the company’s products are subject to seasonality, such as allergy drugs.
Sales and Marketing
In the U.S., Israel and Canada, the company’s sales are primarily generated by its own dedicated sales force. In other countries, the company sells through agents and other distributors. The company’s sales force is supported by its customer service and marketing employees.
In the year ended March 31, 2023, revenue in the U.S. accounted for 63% of total consolidated net sales. In addition to marketing Rx drugs, the company markets its branded and generic OTC products directly to individual customers, and to institutional customers, such as wholesalers, drug chains, food chains, and mass merchandisers. A significant portion of the company’s revenue is derived from sales to a limited number of customers. During the year ended March 31, 2023, the company sold to approximately 179 institutional customers in the U.S.
In the year ended March 31, 2023, sales in Canada accounted for 24% of the company’s total consolidated net sales and Taro Canada sold to approximately 540 institutional customers.
The PMPRB monitors and controls prices of patented drug products marketed in Canada by persons holding, or licensed under, one or more patents.
The marketing, sales, and distribution of Rx pharmaceuticals and OTC products in Israel is closely monitored by the Israeli government. The market for these products is dominated by institutions that are similar to health maintenance organizations in the U.S., as well as private pharmacies. Most of the company’s marketing efforts in Israel focus on selling directly to these groups.
In Israel, the pharmaceutical market generally is divided into two market segments: the private market, which includes drug store chains, private pharmacies, and wholesalers; and the institutional market, which includes Kupat Holim Clalit (the largest health maintenance organization in Israel), other health maintenance organizations, the Israel Ministry of Health, the Armed Forces, and sales to the Palestinian authorities through third parties.
The company has expanded the production capacity of its Israeli, Canadian and Japanese operations to meet anticipated greater demand for its products in future years. In addition, the company utilizes contract manufacturers for certain products to satisfy customer demand in a timely manner.
Research and Development
For the year ended March 31, 2023, the company spent $52.2 million on research and development
Competition
The company competes with companies, such as Novartis AG, Bausch Health Companies Inc., Kenvue, Teva Pharmaceuticals, Viatris Inc., Perrigo Company PLC, Glenmark and Galderma Laboratories.
Regulation
In addition, because the company markets drugs that are classified as controlled substances in the U.S., Canada and Israel, it must meet the requirements of the federal Controlled Substances Act (CSA) and relevant state laws and regulations in the U.S., as well as equivalent laws in Canada and Israel. These regulations include stringent requirements for handing and receipt of controlled substances including import, export, manufacture, storage, security, distribution and dispensing. These requirements include registration/licensing, manufacturing controls (e.g., quotas), import permits/declarations, inventory, recordkeeping, monitoring, disposal, reporting, and security to ensure accountability and prevent diversion of, or the unauthorized access to, the controlled substances in each stage of the production, storage and distribution process. The DEA and state agencies (e.g., relevant state boards of pharmacy) inspect manufacturers, distributors, importers, and exporters that are registered with the U.S. Drug Enforcement Administration (DEA) and licensed by state agencies to review and ensure compliance with the federal CSA and comparable state laws, and DEA regulations with respect to security, record keeping, inventory and reporting prior to issuing a federal controlled substance registration or state license.
History
Taro Pharmaceutical Industries Ltd. was founded in 1959. The company was incorporated under the laws of the state of Israel in 1959.
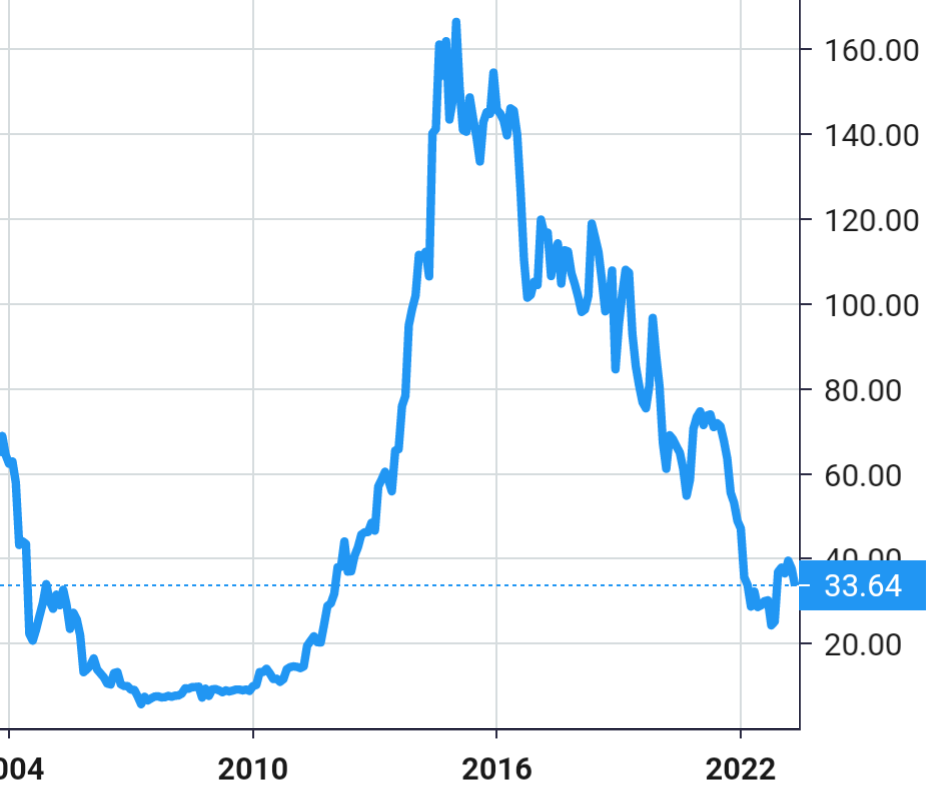
 Stock Value
Stock Value

