About ResMed Inc
ResMed Inc. engages in the development, manufacturing, distribution, and marketing of medical devices and cloud-based software applications that diagnose, treat and manage respiratory disorders, including sleep disordered breathing (SDB), chronic obstructive pulmonary disease (COPD), neuromuscular disease, and other chronic diseases.
Segments
The company operates through two segments, Sleep and Respiratory Care and Software as a Service (SaaS).
The company’s Sleep and Respiratory Care revenue relates primarily to the sale of its products that are therapy-based equipment. Some contracts include additional performance obligations, such as the provision of extended warranties and data for patient monitoring.
The company’s SaaS revenue relates to the provision of software access with ongoing support and maintenance services, as well as professional services, such as training and consulting.
Products

The company’s portfolio of products includes devices, diagnostic products, mask systems, headgear and other accessories, dental devices and cloud-based software informatics solutions.
Devices
The company produces cloud-connected continuous positive airway pressure (CPAP), APAP, bilevel, and ASV devices that deliver positive airway pressure through a patient interface, either a mask or cannula. Its APAP devices, known as AutoSet, are based on a proprietary technology to monitor breathing and can also be used in the diagnosis, treatment and management of obstructive sleep apnea (OSA). During 2017, the company launched AirMini, a small portable CPAP combining the same proven therapy modes used in the AirSense 10 with effective waterless humidification enabling portable convenience. The company commenced a controlled product launch of AirSense 11 in 2021, which was followed by a broader launch throughout 2022. AirSense 11 introduced new features, such as a touch screen, algorithms for patients new to therapy and digital enhancements, such as over-the-air update capabilities. The company also acquired a line of Chinese-developed and manufactured sleep and ventilation devices with the acquisition of Curative Medical in 2016.
CPAP, APAP & Bilevel Products
AirSense 11 (AutoSet, CPAP, Elite): Combining enhanced digital health technology with effective therapy modes, AirSense 11 APAP and CPAP machines are designed to make starting sleep apnea therapy, and adhering to it, easier and convenient. The company’s newest device, AirSense 11 includes new features like Personal Therapy Assistant and Care Check-In designed to provide tailored guidance to PAP users, helping ease them into therapy and comfortable nightly use. Other features include the availability of remote software updates so users can enjoy the latest version of these tools every night.
AirSense 10 (AutoSet, AutoSet for Her, CPAP, Elite, and Card-to-Cloud): AirSense 10 is a widely used series of CPAP and APAP machines, each designed to deliver therapy for a better night’s sleep. Features include a built-in humidifier, Climate Control Auto setting to provide breathing comfort, AutoRamp with sleep onset detection, and expiratory pressure relief (EPR).
AirCurve 10 Bilevel (AirCurve 10 S, AirCurve 10 VAuto, AirCurve 10 ASV, AirCurve 10 VAuto, and Card-to-Cloud): AirCurve 10 bilevel machines include two pressure level settings - A higher pressure when users inhale, and a lower pressure that makes it easier to exhale. AirCurve 10 S and AirCurve 10 VAuto both treat obstructive sleep apnea, while AirCurve 10 ASV treats central sleep apnea. All machines include a built-in humidifier and Climate Control Auto setting to provide breathing comfort.
AirMini portable CPAP: AirMini features the same auto-adjusting therapy modes used in the AirSense 10 Auto. The device also features built-in Bluetooth connectivity and waterless humidification enabled by HumidX technology.
Ventilation Products
Stellar 150: ResMed Stellar 150 ventilator is suitable for invasive and non-invasive ventilation, either at home or in a healthcare setting. It is not a life support ventilator. Stellar 150 also includes iVAPS (intelligent Volume-Assured Pressure Support) 1 technology to adjust to users changing respiratory needs.
Astral 100 and 150: ResMed Astral 100 and Astral 150 provide personalized care every step of the way. With both invasive and non-invasive options, they offer a lightweight design, battery life and adaptive technologies to provide mobility.
AirCurve 10 ST-A: Designed for people with respiratory conditions that affect breathing, such as restrictive lung disorders, severe COPD and hypoventilation, AirCurve 10 ST-A combines controls, an intuitive interface and automatic features to make ventilation therapy effective, comfortable and hassle-free.
Mask Systems, Diagnostic Products, Accessories and Other Products
Mask Systems
Mask systems are one of the important elements of sleep apnea treatment systems. Masks are a primary determinant of patient comfort and as such may drive or impede patient compliance with therapy.
Minimalist: AirFit F30, AirFit P10, and AirFit N30 minimalist masks feature the company’s lightest, lowest profile designs. The features of these masks are focused on minimizing contact with the patient’s face to reduce red marks and irritation.
Freedom: AirFit N30i, AirFit P30i, and AirFit F30i freedom masks, which feature top-of-head tubing design allowing flexibility to switch sleep positions.
Ultra Soft: The AirTouch F20 and AirTouch N20 masks feature a soft and breathable AirTouch cushion designed to enhance CPAP mask comfort.
Universal Fit: AirFit F20 and AirFit N20 masks are designed to fit a range of faces due to the InfinitySeal silicone cushion that adapts to facial contours, which increases comfort, improves the fit and reduces leakage.
Diagnostic Products
The company markets sleep recorders for the diagnosis and titration of sleep apnea in sleep clinics, hospitals and at home. These diagnostic systems record relevant respiratory and sleep data, which can be analyzed by a sleep specialist or physician who can then tailor an appropriate OSA treatment regimen for the patient.
ApneaLink Air: A portable diagnostic device that measures oximetry, respiratory effort, pulse, nasal flow and snoring. Works with AirView Diagnostics to provide comprehensive diagnostic solution to clinicians.
NightOwl: A portable, cloud-connected, fully disposable diagnostic device that measures AHI based on peripheral arterial tone (PAT), actigraphy, and oximetry over several nights to capture variability and help avoid misdiagnosis.
Connected Solutions and Other Products
The company has a suite of products that are designed to allow fewer professionals to manage more patients and empower patients to track their own health outcomes. It is expanding its cloud-based patient management and engagement platforms, such as AirView, enabling remote monitoring, over-the-air trouble shooting and changing of device settings, U-Sleep enabling automated patient coaching through a text, email or interactive voice phone call and myAir, a patient engagement application that provides sleep data and a daily score based on their previous night’s data.
AirView: A cloud-based system enabling remote monitoring and changing of patients’ device settings. AirView also makes it easier to simplify workflows and collaborate across the patient’s care network.
myAir: A personalized therapy management application for patients with sleep apnea providing support, education and troubleshooting tools for increased patient engagement and improved compliance.
U-Sleep: A compliance monitoring solution that enables home medical equipment (HMEs) to streamline their sleep programs to achieve better business and patient outcomes.
Connectivity Module: A module providing cellular connection between the company’s compatible ventilation devices (e.g., Astral, Stellar) and its AirView system.
Propeller: Propeller's inhaler sensors track medication usage and pair with a companion smartphone application, giving people with asthma or COPD a better understanding of their disease and promoting increased adherence to treatment. The Propeller Provider Portal gives clinicians the information they need to make better treatment decisions.
SaaS Products
The company supplies out-of-hospital software products designed to support the professionals and caregivers helping people stay healthy in the home or care setting of their choice.
Brightree Solutions: Brightree enables out-of-hospital care organizations to improve their business performance and deliver better health outcomes. Brightree provides solutions and services for thousands of organizations in home medical equipment and pharmacy, orthotic and prosthetic, and home infusion.
MatrixCare Solutions: MatrixCare’s electronic health record (HER) software as a service solutions are used by skilled nursing and senior living providers, life plan communities (CCRCs), and home health and hospice organizations to improve efficiencies and promote a better quality of life for the people they serve.
HEALTHCAREfirst solutions: HEALTHCAREfirst offers electronic health record (EHR), software, billing and coding services, and advanced analytics that enable home health and hospice agencies to optimize their clinical, financial and administrative processes.
Product Development and Clinical Trials
The company’s product development and clinical trial efforts focus on not only improving its current product offerings and usability, but also expanding into new product applications.
Strategy
The key elements of the company's strategy include to continue product development and innovation in sleep apnea and respiratory care products; broaden its digital health technology foundation; expand SaaS solutions in out-of-hospital care settings; expand geographic presence; increase public and clinical awareness; expand into new clinical applications; and leverage the experience of its management team.
Sales and Marketing
The company markets its products in approximately 140 countries through a network of distributors and its direct sales force.
The United States, Canada and Latin America: The company’s products are purchased by a home healthcare dealer who then sells the products to the patient. In the United States, Canada and Latin America, the company’s sales and marketing activities are conducted through a field sales organization made up of regional territory representatives, program development specialists and regional sales directors. The company’s field sales organization markets and sells products to home healthcare dealer branch locations throughout the United States, Canada and Latin America.
The company also markets its products directly to physicians and sleep clinics. Patients who are diagnosed with OSA or another respiratory condition and prescribed its products are typically referred by the diagnosing physician or sleep clinic to a home healthcare dealer to fill the prescription. The home healthcare dealer, in consultation with the referring physician, would assist the patient in selecting the equipment, fit the patient with the appropriate mask and set the device pressure to the prescribed level.
The company’s SaaS solutions are sold to providers of healthcare in various out-of-hospital settings. It markets and sells its Brightree business management software and service solutions to out-of-hospital providers in the U.S and its primary markets are home medical equipment, pharmacy, home infusion, orthotics and prosthetics. The company’s sales activities for Brightree products are conducted through a sales organization made up strategic account managers, sales engineers and sales directors. It develops, markets and sells its MatrixCare care management and related ancillary solutions to U.S.-based out-of-hospital providers and its primary markets are senior living, skilled nursing; life plan communities; home health, home care, and hospice agencies, as well as related accountable care organizations. Its MatrixCare management solutions are primarily sold through direct sales and ancillary solutions are sold both through direct sales and channel partners.
Combined Europe, Asia and other markets: The company markets its products in most major countries in combined Europe, Asia and other markets. It has wholly-owned subsidiaries in Australia, Austria, China, Czech Republic, Denmark, Finland, France, Germany, India, Ireland, Japan, Korea, Netherlands, New Zealand, Norway, Poland, Sweden, Switzerland, Taiwan, Thailand, and the United Kingdom. The company uses a combination of its direct sales force and independent distributors to sell its products in combined Europe, Asia and other markets. In countries where the company sells its products direct, a local senior manager is responsible for direct national sales. In various countries it sells its products to home healthcare dealers or hospitals who then sell the products to the patients. In Germany, Australia, New Zealand, and South Korea, the company also operate a home healthcare company, in which it provides products and services directly to patients.
Patents and Proprietary Rights and Related Litigation
Through its various subsidiaries, as of June 30, 2022, the company owned or had licensed rights to approximately 9,100 pending, allowed or granted patents and designs. Of its patents, 565 U.S. patents and 1,435 foreign patents are due to expire in the next five years.
Research and Development
For the year ended June 30, 2022, the company invested $253.6 million on research and development activities.
Government Regulations
The company’s products are subject to regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Its products marketed in the United States are marketed pursuant to 510(k) pre-marketing clearances and are either Class I or Class II devices.
The company is required to adhere to applicable regulations setting forth detailed cGMP requirements, as set forth in the QSR, which require, manufacturers, including third-party manufacturers, to follow design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process.
Where appropriate, the company’s products commercialized in Europe are CE marked and classified as either Class I or Class II. The company’s devices are sold in multiple countries and need to be registered with local regulatory bodies, such as the Therapeutic Goods Administration in Australia, Health Canada in Canada, and CFDA in China.
In some of the company’s operations, such as those involving its cloud-based software digital health applications, the company is a business associate under the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA) and therefore required to comply with the HIPAA Security Rule, Breach Notification Rule and certain provisions of the HIPAA Privacy Rule; and is subject to civil and criminal penalties for failure to do so.
Competition
The company’s primary Sleep and Respiratory Care competitors include Philips BV; Fisher & Paykel Healthcare Corporation Limited; DeVilbiss Healthcare; Apex Medical Corporation; BMC Medical Co. Ltd.; and regional manufacturers.
History
ResMed Inc. was founded in 1989. The company was incorporated in 1994.

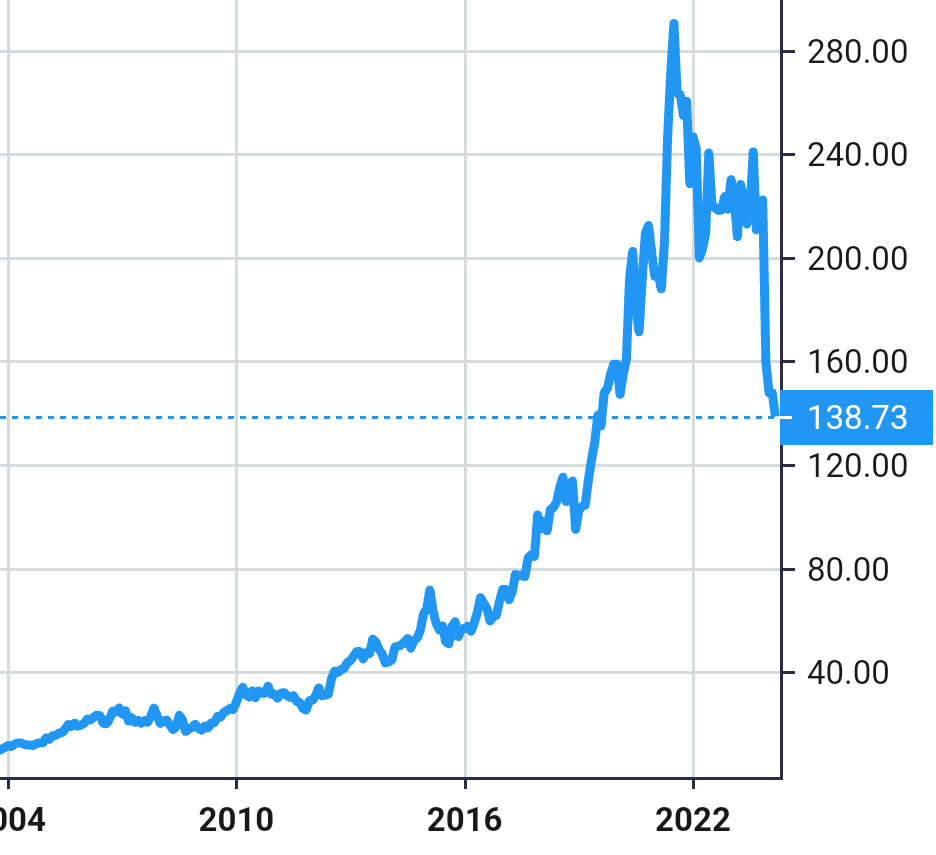
 Stock Value
Stock Value

