About MaxCyte
MaxCyte, Inc. operates as a leading commercial cell engineering company. The company focuses on providing enabling platform technologies to advance the discovery, development and commercialization of next-generation cell therapeutics and to support innovative cell-based research and development. The company has developed and commercialized its proprietary Flow Electroporation platform, which facilitates complex engineering of a wide variety of cells.
The company’s ExPERT platform, which is based on its Flow Electroporation technology, has been designed to address this rapidly expanding cell therapy market and can be utilized across the continuum of the high-growth cell therapy sector, from discovery and development through commercialization of next-generation, cell-based medicines. The ExPERT family of products includes four instruments, which the company calls the ATx, STx, GTx and VLx, as well as a portfolio of proprietary related disposables and consumables. The company launched the ExPERT VLx instrument for very large-scale cell engineering in September 2022. The company’s disposables and consumables include PAs designed for use with its instruments, as well as accessories supporting PAs, such as electroporation buffer solution and software protocols. The company has garnered meaningful expertise in cell engineering via its internal research and development efforts, as well as its customer-focused commercial approach, which includes a growing application scientist team. The platform is also supported by a robust intellectual property portfolio with more than 150 granted U.S. and foreign patents and more than 95 pending patent applications worldwide.
From leading commercial cell therapy drug developers and top biopharmaceutical companies to top academic and government research institutions, including the U.S. National Institutes of Health (NIH), the company’s customers have extensively validated its technology. The company’s existing customer base ranges from large biopharmaceutical companies, including all of the top 10, and 20 of the top 25, pharmaceutical companies based on 2021 global revenue, to hundreds of biotechnology companies and academic centers focused on translational research.
Technology Platform
The foundation of the company’s technology is its proprietary and patented Flow Electroporation platform, which it has developed and optimized for more than 20 years. Electroporation can be applied to almost any eukaryotic cell type to deliver a broad range of molecules, including DNA, human messenger RNA (mRNA), siRNA and proteins. The company’s proprietary Flow Electroporation platform is fully scalable and can support small-scale research and development through large-scale cell engineering for the development of commercial therapeutics.
The company’s technology platform is marketed under the ExPERT brand. The value of the company’s ExPERT brand starts with Efficiency—with high delivery Efficiency, users can achieve Potency, with high Potency, users improve their chances of therapeutic Efficacy, and if this can be repeated, Reproducibly from patient to patient, users have a successful Therapy. By delivering high efficiency at any scale, the ExPERT platform is designed to improve the company’s customer’s ability to achieve the required therapeutic index, enabling accelerated, cost-efficient translation of complex cellular therapies from research to the clinic.

The company’s ExPERT platform consists of four instruments, the ATx, STx, GTx and VLx, which use a broad range of PAs, or disposables, of different volumes to enable scalable electroporation from tens of thousands to billions of cells to facilitate the translation of complex cellular therapies from concept to the clinic, in support of the intended therapeutic commercialization. The company’s family of instruments and disposables have been designed to support scale-up for cell therapy development.
ExPERT Platform
The company’s Flow Electroporation Technology was designed to meet the stringent demands of clinical use—namely, the ability to safely and reproducibly modify a broad range of primary human cells with high efficiency, low cytotoxicity, and at the scale required to enable the treatment of patients across a diverse range of diseases.
The combination of the ExPERT instruments, associated disposables and universal electroporation buffer provides researchers, production scientists, and cGMP facilities with a solution to transfect cells with high efficiency, viability and consistency, which are the three attributes that are consistently ranked by the company’s customers as the top requirements when choosing a cellular or gene engineering platform for clinical use. The company’s instruments are sold or licensed for research or clinical use, while the associated disposables and electroporation buffer are sold to support pre-clinical research and development work and are compatible for integration into cGMP manufacturing environments.
In addition, the company has implemented a global scientific and regulatory support strategy for its customers that is designed to accelerate clinical development and streamline the regulatory submission process.
The company’s ExPERT platform offers a compelling value proposition to its academic and biopharmaceutical customers due to the ability to use its technology to deliver almost any molecule into almost any cell type, including hard-to-transfect human primary cells, while maintaining high cell viability and function; the capacity to introduce larger and more diverse molecules, as well as multiple payloads, which exceeds the capabilities of other intracellular delivery technologies, such as viral vectors; and the flexibility to scale up from research to cGMP, manufacturing on a single platform—enabling the engineering of cells ranging from tens of thousands of cells to tens of billions of cells in a single transfection run in 30 minutes or less.
The company’s ExPERT intracellular delivery platform provides value across numerous applications in the life sciences market, including research, discovery, development, and manufacturing of next-generation, cell-based therapeutics, as well as in biomanufacturing, such as transient protein production for drug discovery and manufacturing of other proteins, including biological therapeutics, viral vectors and vaccines, and small molecule drug discovery.
The company’s ExPERT technology platform is being used in the clinic to support the development of next-generation cell therapy approaches to treat human disease. Following the successful clinical development leading to FDA approvals of CAR-T cell therapies in blood-based cancers, developers have focused on improving efficacy, lowering the cost of manufacturing and/or expanding engineered cell therapies into new indications, such as solid tumors, as well as autoimmune and neurogenerative diseases.
Agreements with Customers
The company has a diverse portfolio of clinical partners and licensees that mirror the overall next-generation engineered ex vivo cell therapies. While difficult to predict given uncertainty around regulatory approvals and clinical risk, according to Evaluate Pharma, a provider of commercial intelligence and predictive analytics to the pharmaceutical industry, the first next-generation ex vivo cell therapies using non-viral approaches could be approved in the United States as early as 2023.
The company’s platform’s ability to engineer a diversity of cell types (including CAR-T, chimeric antigen receptor Natural Killer cells (CAR-NK/NK), T cell receptor (TCR) and stem cells) and cell sources (autologous and allogeneic) enhances its opportunity by potentially providing SPL partnership revenues regardless of which approaches advance in the coming years. Additionally, the company’s instruments and platform have been used in over 40 clinical trials to date for drugs being developed to treat a variety of indications, from hematological malignancies to solid tumors to inherited genetic disorders.
Products
ExPERT Instruments
The ExPERT instrument family was designed to provide a single unifying technology that can be used from concept to clinic, with both the research and clinical versions of the instrument incorporating the same underlying technology and protocols. The company’s customers have a choice of three different instrument versions that are standardized on the same technology to deliver equivalent high performance—the ATx, STx and GTx, as well as a portfolio of proprietary related disposables and consumables. In September 2022, the company introduced a fourth instrument, the ExPERT VLx, for large-scale cell engineering. Customers can start with the lower to medium scale research instrument (ATx) and then scale up to the clinical version (GTx) without the need for re-optimization or re-validation. The STx provides the same scale as the GTx but is used for drug discovery applications, such as preclinical monoclonal antibody production, and not for human therapeutic use. The STx is not covered by the company’s FDA Master File or its Technical Files.
All the company’s instruments have been designed to provide customers with the key features required for a scalable high-performance transfection solution. Each of the company’s ExPERT instruments are benchtop with the same small footprint and have integrated touch screens with an intuitive Graphical User Interface (GUI), designed for simple training and operation. To support use in the cGMP suite for clinical manufacturing, the company’s GTx ExPERT software is network capable to enable upload of electronic batch records to a local shared drive and has a software intermediary to facilitate integration and automated data transfer to cloud-based data management solutions. The company has integrated hardware and software design solutions, manufactured under cGMP, that are tailored for use in cGMP manufacturing of clinical products for advanced cellular therapies.
The company’s ExPERT ATx static electroporation instrument is a research focused, high performance electroporation platform for small to medium scale transfection. The ATx instrument delivers high efficiency and viability at research scale and can utilize its range of PAs capable of transfecting from 75,000 up to 700 million cells. Additionally, the company’s ATx instrument is compatible with all of its static PAs, which can also be used on its GTx instrument, allowing for a seamless transition to its clinical cGMP-compatible platform. The ATx is designed and used by the company’s customers for early design of experiment and process optimization at small scale to minimize cell acquisition and reagent costs.
The company’s ExPERT STx, which is used in the field of protein production, as well as other drug discovery applications, also incorporates its proprietary Flow Electroporation Technology for high yield transient expression of complex proteins, viral vectors, vaccines and biologics. The company’s STx instrument has high efficiency and can rapidly transfect from 75,000 up to 20 billion cells.
The ExPERT GTx incorporates the company’s proprietary Flow Electroporation Technology for use in the cGMP manufacturing of cellular therapies for use in the clinic.
The GTx integrates several design features that are critical for use in a cGMP setting, such as barcode reading capability to maintain positive identification of patient samples, 21 CFR Part 11 compatible software and networking capability for automated uploading of electronic batch records to either a central server or to a cloud-based data management platform. The GTx enables closed sample processing, on a system compatible with integration into cGMP manufacturing environments, and that has an established regulatory path supported by the company’s FDA Master File and Technical Files.
The ExPERT VLx Large-Scale Transfection System is a cGMP compliant instrument specifically designed for very large volume cell-engineering. Using proprietary Flow Electroporation Technology, the VLx supports the ability to transfect up to 200 billion cells in less than 30 minutes—10 times the capacity of the STx and GTx. This system is designed for the rapid and large-scale production of recombinant proteins, monoclonal antibodies, viral vectors, vaccines, virus-like particles (VLPs), and allogeneic cell therapies. We launched the VLx under the ExPERT brand in September 2022 to provide customers with an easy to use, large-scale system that incorporates the benefits of the ExPERT platform for large-scale bioprocessing.
Disposables—Processing Assemblies (PAs)
The company’s range of disposable PAs is an important differentiator for it. The company has developed a broad range of PAs that are specially designed to process and electroporate the user’s chosen quantity of cells. Each PA contains two electrodes, between which a medical-grade gasket is sandwiched that has a unique well design consistent with the processing volume required and to allow maximum retrieval of cells. The company’s PAs are capable of electroporating cell volumes from small to large scale, in single and multi-well formats, for both research and clinical use. Cells are placed into the sample bag in large scale PAs, or into the well or wells in small scale PAs, and the PA is then connected to the instrument for processing. The instrument touch screen allows the operator to select the desired cell protocol that encodes the electroporation parameters, select the type of PA to be used and enter any sample specific information. Once the sample information has been entered, the operator will touch the Start Processing icon on the user interface and the sample will be rapidly processed. Larger volumes of cells are accommodated by larger capacity PAs and a set of simple software commands through the intuitive GUI.
The company’s ExPERT system uses two PA designs — a static cuvette used for smaller cell volumes (from 75,000 cells up to 200 million cells) and a cartridge design that is used for both static and Flow Electroporation for larger cell volumes (700 million up to tens of billions of cells). The Flow Electroporation PA (Flow PA) allows for processing of cellular volumes ranging from 10 mL to 100 mL and up to tens of billions of cells. The Flow PA consists of bags and associated tubing, made from medical grade materials, that are connected to the electroporation cartridge. Users transfer their cells and loading molecules to the sample bag, and the pump on either the GTx or STx instrument pumps a fixed volume of cells into the cartridge chamber where they are electroporated. Once the electroporation is complete, the cells are pumped to the collection bag and the chamber is filled with the next volume of cells for electroporation. This process is repeated until the entire sample volume is processed. The maximum volume of 100 mL of cells can be processed in approximately 15–20 minutes.
The company has conducted extensive end-user research to continue improving the design of the PAs and the range of products available. The company launched the ExPERT cuvette in 2020 based on customer feedback, which incorporated a new design to improve handling and ease of use and have continued to expand the availability the ExPERT PA portfolio design. The company has also expanded its portfolio of multi-well cuvettes, which reduce manual handling and improve productivity in the lab, with the launch of its R-50x8. The R-50x8 is an 8-well cuvette capable of processing up to 225,000 cells in each well. By enabling eight samples to be processed in the same cuvette, a more efficient process can be achieved by users. The company plans to continue to support customers using legacy processing assemblies (Pas) until they transition to its ExPERT products.
In 2022, the company added the R-20K Flow Electroporation Processing Assembly for its STx and GTx platforms, which can process between 5 mL and 20 mL sample volumes, which can accommodate between 200 million and up to 4 billion cells. The R-20K assembly will allow clients to develop therapies at small or mid-scale volumes with improved cell recovery in a closed process adaptable format to assist in de-risking their manufacturing process at the electroporation step. To further support its customers’ sterile closed process workflows, the company also introduced ‘Closed Process Electroporation Buffer’ products in two volume sizes, 500 mL and 1 Liter, which allow for the addition of its proprietary electroporation buffer to concentrated cell samples before electroporation. For its VLx Platform the company introduced the R-1L Flow Electroporation Processing Assembly, which can process in 30 minutes or less between 100 mL and 1 Liter sample volume accommodating up to 200 billion cells in a single run. The R-1L assembly allows for large volume sample processing that can be adapted to a closed and sterile workflow for continuous end-product production.
The company is committed to strategically investing in improvements in the PA design and range of products to ensure that customers have solutions that address all of their volume and use requirements, in both research and clinical settings, including development of advancements for PA that support the VLx.
Supporting Products
The company’s proprietary electroporation buffer, a balanced salt solution that protects cells during transfection, is formulated for use with all its instrument platforms and PAs. This consumable is used for all cell types, eliminating the need to change buffers as users switch protocols, cell types or scale up. The buffer is made in a cGMP facility, is fully chemically defined and is free of human or animal components, and is tested to meet technical, sterility and endotoxin specifications. This buffer formulation is a key contributing factor, in combination with instrument and PA design features, to the flexibility, high efficiency and viability that can be achieved by customers across the broad range of cell types processed using its platform.
Sales and Marketing
The company follows a direct sales model in North America, the United Kingdom, and Europe, while also selling through third-party distributors in Asia and some regions of Europe. As of December 31, 2022, the company had over 25 field sales and application scientists located in the United States, the United Kingdom, and several regions in Europe and Asia. Since the commercial launch of the company’s first Flow Electroporation instrument, the installed base of its instruments has grown to more than 600 instruments globally.
The company’s sales force and field application scientists and international partners inform its current and potential customers of current product offerings, new target applications and advances in its technologies and products. As its primary point of contact in the marketplace, the company’s field teams focus on delivering a consistent marketing message and high level of customer support, while also working to help it better understand the evolving market and customer needs. The company intends to expand its sales, support, and marketing efforts in regions such as the Asia-Pacific region. The company uses distributors in countries in these regions, such as in China and Japan, supplemented by dedicated its team members, and continuously assess the need for direct sales and local support personnel to supplement its distributors’ resources. As it expands into a new geography, the company generally relies initially on third-party distributors until it is able to recruit a direct sales force, field application scientists and business development resources in the country or region.
The company’s business model is focused on identifying new applications in cell engineering to enable its customers to develop better medicines and maximize use across its customers’ value chains. This is enabled through customer partnerships that allows it to further understand the critical applications for its technology and inform its future developments and market expansion.
Research and Development
The company’s research and development expenses totaled $19.5 million for the year ended December 31, 2022.
Intellectual Property
The company focuses on obtaining protection for its non-viral delivery platform to the extent possible, particularly in the United States and other key jurisdictions of commercial value. As of March 8, 2023, the company has more than 150 granted U.S. and foreign patents, including in foreign jurisdictions, such as Australia, Canada, Japan, China, South Korea and certain countries in Europe, as well as over 95 pending patent applications worldwide. The main focus of the company’s patent coverage is to protect its Flow Electroporation process, as well as the processing assemblies/disposables, control and process elements, and methods/applications of using its non-viral delivery platform. The company’s patent portfolio provides protection over its instruments and related methods through at least 2028 and over its electroporation applications and methods through 2034. The company is also working to secure design protection of the ExPERT system, which has the potential to provide protection through at least 2036.
As of March 8, 2023, the company owned 17 registered trademarks in the United States, 191 registered foreign trademarks, 13 pending U.S. trademark applications, and more than 31 pending foreign trademark applications. The company’s registered trademarks and pending trademark applications include trademarks for its company name, a stylized version of ExPERT, and its logo. In order to supplement protection of its brand, the company has also registered several internet domain names.
Government Regulation
The company’s biopharmaceutical and life sciences customers are subject to extensive regulations by the FDA and equivalent regulatory authorities in other countries, regarding the conduct of preclinical studies and clinical trials, in the manufacture of product candidates and products for use in humans (i.e.,Good Manufacturing Practice laws and regulations) and the marketing authorization and commercialization of biological drug products.
In order to support its customers’ use of its platform, the company has voluntarily submitted a Master File to the FDA, Center for Biologics Evaluation and Research and Master Files or Technical Files to comparable regulatory authorities in other jurisdictions, including Canada, Japan, the United Kingdom and Austria, and provide nonexclusive Letters of Authorization to the Master or Technical Files under contractual agreements with its customers. The company has also discussed the potential to submit a Master File with the Therapeutic Goods Administration in Australia. continuously update the Master and Technical Files in order to support the regulatory activities of the company’s customers.
History
MaxCyte, Inc. was founded in 1998. The company was incorporated in 1998.

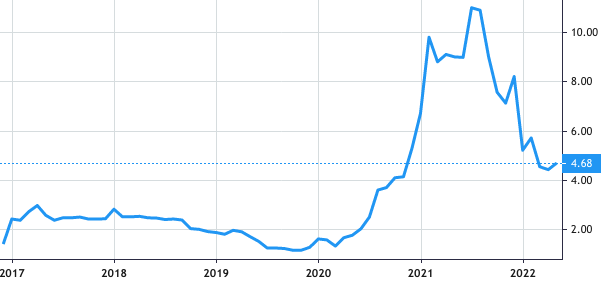
 Stock Value
Stock Value

