About Codexis
Codexis, Inc. operates as an enzyme engineering company.
The company is leveraging its proprietary CodeEvolver technology platform to discover, develop and enhance novel, high performance enzymes and other classes of proteins. The enzymes the company produces solve for real-world challenges associated with small molecule pharmaceuticals manufacturing, nucleic acid synthesis and genomic sequencing, and – as biotherapeutic candidates – they have the potential to treat challenging diseases. The company’s unique enzymes drive improvements, such as higher yields, reduced energy usage and waste generation, improved efficiency in manufacturing, greater sensitivity in genomic and diagnostic applications, and potentially more efficacious therapeutics.
The company’s novel biotherapeutics business includes a diverse pipeline of product candidates in clinical and preclinical development. Its initial biotherapeutic product candidates include enzymes that are orally administered for function in the gastrointestinal tract (GI), such as the company’s partnered product candidates CDX-7108 for the treatment of exocrine pancreatic insufficiency and CDX-6114 for the treatment of phenylketonuria, which are both in Phase 1 clinical trials. The company has also engineered a series of transgenes that code for enzymes that may be used as gene therapies to treat rare lysosomal storage disorders with the company’s partner Takeda, such as Fabry Disease and Pompe Disease, as well as a blood factor disorder.
The company’s performance enzymes business consists primarily of two focus areas: biocatalysts for the sustainable manufacturing of pharmaceuticals and enzymes for life science applications, including genomic sequencing and nucleic acid synthesis. In the company’s pharmaceutical manufacturing business, it utilizes its CodeEvolver platform to develop optimized enzymes that are used by some of the world’s largest pharmaceutical companies to reduce their costs and improve the efficiency and productivity of their manufacturing processes for some small molecule therapeutics. In life science markets, the company uses its platform technology to develop enzymes for customers using next generation sequencing (NGS), a parallel sequencing technology used to identify genomic information in the study of biological systems, and PCR/qPCR for in vitro molecular diagnostic and molecular biology research applications, as well as for synthesis of nucleic acids, such as DNA/RNA.
Novel Biotherapeutics
The company is developing a diverse pipeline of product candidates in its novel biotherapeutics business. These product candidates, which are in clinical and preclinical development, range from orally delivered enzymes to engineered transgenes for delivery as gene therapies that have the potential to address a range of diseases with high unmet patient need. Each of the company’s product candidates is discovered utilizing its proprietary CodeEvolver protein engineering platform.

Partnered Oral Enzyme Programs
CDX-7108 for the Treatment of Exocrine Pancreatic Insufficiency
Under a Strategic Collaboration Agreement with Nestlé Health Science (‘Nestlé SCA’), the company has collaboratively developed CDX-7108, a potent lipase intended for use as a pancreatic enzyme replacement therapy (‘PERT’). CDX-7108 has been specifically engineered for increased potency as a lipase and also to remain stable in acidic conditions, such as those encountered in the stomach.
Under the Nestlé SCA, the company and Nestlé Health Science are also working on the development of engineered amylase and protease enzymes for possible use with CDX-7108. Nestlé Health Science is dosing patients in a Phase 1b three-party study. The first two parts of the study evaluated the safety, tolerability, and pharmacokinetics (‘PK’) of escalating single and multiple oral doses of CDX-7108 in 48 healthy adult subjects, with no safety issues noted. The third part of the study is evaluating the pharmacodynamics of a single dose of oral CDX-7108 in six enrolled patients with exocrine pancreatic insufficiency (‘EPI’). An interim analysis conducted in January 2023 of five patients who had completed the study at the time showed a clear indication of improved lipid absorption when patients are administered CDX-7108 versus placebo, which supports a path forward together with Nestlé Health Science to further develop CDX-7108, with the potential for the initiation of a Phase 2 study in early 2024.
CDX-6114 for the Treatment of Phenylketonuria
The company internally developed CDX-6114, an enzyme it engineered to be orally administrated for the treatment of phenylketonuria (‘PKU’) in humans. PKU, one of the most common inborn errors of metabolism (‘IEMs’), is a metabolic disorder in which the enzyme that converts the essential amino acid phenylalanine into tyrosine is deficient. As a result, phenylalanine accumulates to toxic levels in the brain, causing serious neurological problems, including intellectual disability, seizures and cognitive and behavioral problems. To avoid toxic levels of phenylalanine in their blood, individuals with PKU must follow a strict, life-long diet that is low in phenylalanine and supplement their diet with a synthetic phenylalanine-free protein supplements to provide them with sufficient nutrients. Maintaining a strict, life-long diet can be challenging for individuals with PKU.
The company has partnered with Nestlé Health Science under a Global Development, Option and License Agreement (‘Nestlé License Agreement’) to further develop CDX-6114. In February 2019, Nestlé Health Science exercised its option under the Nestlé License Agreement to obtain an exclusive license to develop and commercialize CDX-6114. Nestlé Health Science is optimizing the formulation of CDX-6114 to improve performance and the company expects Nestlé Health Science to announce an IND filing and clinical trial initiation in 2023. If this collaboration can successfully demonstrate benefit in PKU patients with CDX-6114, this will inform the company’s decisions around the oral enzyme approach to several other IEMs.
Wholly-owned Oral Enzyme Programs
In the past the company has also worked on internal programs to develop orally administrable enzyme substitution therapy candidates for the treatment of homocystinuria (‘HCU’) and Maple Syrup Urine Disease (‘MSUD’), that the company is considering partnering options for pursuing further development. In addition, the company has a program to develop orally administrable enzyme substitution therapy candidates for the treatment of Celiac Disease (‘CD’).
Gene Therapy
The company has also used CodeEvolver to engineer transgenes that encode for enzymes, which may improve targeting and expression within the body when administered as gene therapies, offering potentially improved therapeutic benefit as compared to current options.
Partnered Gene Therapy Programs
The company’s first significant program involving engineered transgenes commenced in March 2020 when the company entered into a Strategic Collaboration and License Agreement (‘Takeda Agreement’) with Shire Human Genetic Therapies, Inc., a wholly-owned subsidiary of Takeda Pharmaceutical Co. Ltd. (‘Takeda’) pursuant to which the company is collaborating to research and develop transgenes for use in gene therapy delivery technology for rare lysosomal storage disorders, such as Fabry Disease, Pompe Disease, a blood factor disorder, and another lysosomal storage disorder. Of these programs, the Fabry disease program is the most advanced, with a lead candidate identified in investigational new drug (‘IND’) enabling activities. The company has also provided sequences to Takeda for the Pompe program and await updates on preclinical testing and potential IND enabling activities. In May 2021, Takeda elected to exercise their option to initiate an additional program for a certain undisclosed rare genetic disorder and the company received the option exercise fee during the third quarter of 2021.
In addition to the company’s partnered gene therapy programs, the company continues to explore the possible application of its CodeEvolver technology to develop therapeutic options for devastating diseases, as well as to develop and test the company’s own proprietary gene therapy delivery mechanisms.
Performance Enzymes
The company’s performance enzymes business consists primarily of two focus areas, pharmaceutical manufacturing and life science products.
Pharmaceutical Manufacturing
The company’s pharmaceutical manufacturing customers, which include many large global pharmaceutical companies, partner with it to develop optimized enzymes for use as biocatalysts, meeting precisely defined criteria.
As of December 31, 2022, the company was selling biocatalysts to pharmaceutical manufacturers for 18 therapeutic drugs that were approved for commercial sales.
Of particular note for 2022, in July 2022, the company announced that it and Pfizer had entered into an agreement to supply Pfizer with CDX-616, a proprietary high performance enzyme used to manufacture a critical intermediate for nirmatrelvir, an active pharmaceutical ingredient in PAXLOVID, Pfizer’s antiviral therapeutic, which is authorized for emergency use by the FDA for the treatment of mild-to-moderate COVID-19 in people at high risk of progression to severe illness and authorized or approved by other regulatory authorities across the globe. While the company has generated significant revenue from supplying CDX-616 to Pfizer, there is no future binding commitment for them to purchase any particular quantity or quantities of CDX-616 from the company.
The company regularly sells biocatalysts, at multi-kilograms to metric tons per annum scale, that have already been engineered, scaled up, and installed in a customer’s commercial process. For example, in addition to Pfizer, the company sells biocatalysts to Merck for their manufacture of sitagliptin, the active ingredient in JANUVIA, to Urovant and Kyorin for the manufacture of vibregon, the active ingredient in Urovant’s GEMTESA and Kyorin’s BEOVA, products for the treatment of overactive bladder, as well as supporting other products and customers for which public disclosures have not been made.
In addition to these larger volumes of biocatalysts that are sold for the company’s customers’ ongoing commercial requirements, it sells lesser quantities of enzymes for use in a customer’s developmental, qualification or regulatory approval operations. As of December 31, 2022, 18 drug candidates in Phase 2 and Phase 3 clinical trials use enzymes engineered using CodeEvolver technology (either by Codexis or by the company’s platform licensing partners) in their chemistry, manufacturing and control processes. This pipeline of potential approvals reinforces the company’s confidence in its ability to continue to grow this business over time.
Finally, the company sells even smaller quantities of enzymes (typically grams to multi-kilograms scale) to customers for experimental, testing and qualification purposes, or as part of an enzyme engineering project.
In addition to the sale of biocatalysts, the company offers research and development partnerships to its customers. These research and development activities are typically governed by collaboration agreements, which often contain research fee payments and intellectual property provisions, under which the company screens and/or engineers biocatalysts for customers in connection with their process development efforts.
The company also has licensed its CodeEvolver enzyme engineering technology platform to pharmaceutical companies to help them develop custom-designed enzymes that are highly optimized for efficient manufacturing processes. As of December 31, 2022, the company entered into platform technology licensing agreements with each of GlaxoSmithKline Intellectual Property Development Limited, a subsidiary of GlaxoSmithKline plc (‘GSK’), Merck, Sharp & Dohme (‘Merck’) and Novartis Pharma AG (‘Novartis’).
Life Sciences
The company also applies its CodeEvolver technology to develop enzymes for customers using NGS and PCR/qPCR for in vitro molecular diagnostic and molecular biology research applications, as well DNA/RNA synthesis applications.
In December 2019, the company entered into a license agreement to provide Roche Sequencing Solutions, Inc. (‘Roche’) with an improved DNA ligase (EvoT4 DNA ligase) for NGS library prep, which continues to progress towards commercialization in new NGS kits.
In June 2020, the company entered into a co-marketing and enzyme supply collaboration agreement with Alphazyme LLC for the production and co-marketing of enzymes for life science applications. Since then, this collaboration has enabled the commercialization of Codex HiFi DNA Polymerase, Codex HiFi Hot Start DNA Polymerase, Codex HiFi Hot Start 2X NGS Mix, Codex HiCap RNA Polymerase, Codex HiFi UL DNA Polymerase, and Codex HiTemp Reverse Transcriptase. Development of other novel enzymes for life science applications continues.
Also, in June 2020, the company entered into a Master Collaboration and Research Agreement with Molecular Assemblies, Inc. (‘MAI’) (the ‘MAI Agreement’) pursuant to which the company is leveraging its CodeEvolver platform technology to improve the DNA polymerase enzymes that are critical for enzymatic DNA synthesis.
In April 2022, the company and MAI announced that, using its CodeEvolver platform technology, the company had developed a novel, engineered terminal deoxynucleotidyl transferase (‘TdT’) enzyme, which would enable MAI’s Fully Enzymatic Synthesis (‘FES’) technology that produces highly pure, sequence-specific DNA on demand. In August 2022, the company and MAI announced that it had entered into a Commercial License and Enzyme Supply Agreement with MAI (the ‘MAI Supply Agreement’) under which Codexis shall manufacture and sell the TdT enzyme to MAI for use in native DNA synthesis.
In March 2022, the company announced the initiation of a strategic partnership with seqWell Inc., a developer of transformative library preparation products for demanding genomics plan application, which included an investment to accelerate the commercialization of seqWell’s genomics workflow solutions. The company and seqWell plan to collaborate on using its CodeEvolver platform technology for enzyme optimization with seqWell’s growing portfolio of genomics workflow and library preparation products.
Strategy
The company’s strategy is to grow its revenues, profits, and stockholder value by leveraging its CodeEvolver enzyme engineering technology platform in the following ways: creating and advancing novel biotherapeutic drug candidates; growing its pharmaceutical manufacturing business; and developing high-performance enzymes for use in life science applications and nucleic acid synthesis.
Strategic Collaborations
Biotherapeutics
Nestlé Health Science
In October 2017, the company entered into the Nestlé License Agreement with Nestlé Health Science pursuant to which the company granted to Nestlé Health Science, under certain of its patent rights and know-how: an option to obtain an exclusive, worldwide, royalty-bearing, sublicensable license to develop and commercialize certain products (each, a ‘Product’) based on CDX-6114 and the company’s other therapeutic enzyme product candidates covered by specified patent applications for the treatment of hyperphenylalaninaemia (HPA) (also referred to as PKU); and an exclusive right of first negotiation (the ‘Right of First Negotiation’) for a period of five years to obtain an exclusive worldwide license to develop and commercialize up to two enzymes discovered by the company for use in the field of the prevention, diagnosis, treatment and management of inborn errors of amino acid metabolism.
In February 2019, Nestlé Health Science exercised its option to obtain an exclusive, worldwide, royalty-bearing, sub-licensable license for the global development and commercialization of CDX-6114 for the management of PKU. Upon exercising its option, Nestlé Health Science assumed all responsibilities for future clinical development and commercialization of CDX-6114, with the exception of the completion of an extension study, CDX-6114-004, which was substantially completed in the fourth quarter of 2019. The parties established a patent committee to discuss strategies and coordinate activities for the patents related to CDX-6114 and product containing CDX-6114, and the company will jointly own all inventions and information that result from each party’s activities performed under the Nestlé License Agreement.
In October 2017, the company entered into the Nestlé SCA pursuant to which the company and Nestlé Health Science are collaborating to leverage the CodeEvolver enzyme engineering technology platform to develop novel enzymes for Nestlé Health Science’s established Consumer Care and Medical Nutrition business areas. The term of the Nestlé SCA has been extended through December 2023 with automatic renewal through December 2024.
In January 2020, the company entered into the Nestlé development agreement (the ‘Nestlé DA’) pursuant to which the company and Nestlé Health Science are collaborating to advance CDX-7108 into preclinical and early clinical studies. CDX-7108 is the lead candidate discovered under the Nestlé SCA targeting exocrine pancreatic insufficiency. The term of the Nestlé DA has been extended through December 2023 with automatic renewal through December 2024.
Shire Human Genetic Therapies/Takeda Pharmaceutical
In March 2020, the company entered into the Takeda Agreement with Takeda pursuant to which the company is collaborating to research and develop protein sequences for use in gene therapy products for certain diseases (each, a ‘Field’) in accordance with each applicable program plan (each, a ‘Program Plan’).
The company granted to Takeda an exclusive, worldwide, royalty-bearing, sublicensable license to use the protein sequences and their corresponding nucleic acid sequences to develop, manufacture and commercialize the applicable products in the applicable Field. The company also granted to Takeda a limited non-exclusive, worldwide, sublicensable license to research the protein sequences within or outside the applicable Fields; and to research the products outside of the applicable Fields, which such rights exclude Takeda's right to perform any IND-enabling activities. The licenses to research the Protein Sequences expire after a pre-determined period of time.
Licensing CodeEvolver Enzyme Engineering Technology Platform
GlaxoSmithKline
The company entered into its first CodeEvolver enzyme engineering Platform Technology Transfer, Collaboration and License Agreement (GSK CodeEvolver Agreement) in July 2014 with GlaxoSmithKline Intellectual Property Development Limited, a subsidiary of GSK, pursuant to which the company granted GSK a non-exclusive, worldwide license to use the company’s CodeEvolver enzyme engineering technology platform in the field of human healthcare for its internal development purposes.
Under the GSK CodeEvolver Agreement, the company licensed and transferred its certain patents, patent applications and know-how from the company’s CodeEvolver enzyme engineering technology platform to GSK, completing the transfer in April 2016.
Merck
In August 2015, the company entered into a CodeEvolver Platform Technology Transfer and License Agreement (the ‘Merck CodeEvolver Agreement’) with Merck. The Merck CodeEvolver Agreement allows Merck to use the company’s proprietary CodeEvolver enzyme engineering platform technology in the field of human and animal healthcare.
Under the terms of the Merck CodeEvolver Agreement, the company granted to Merck an exclusive license under certain patents, patent applications and know-how from its CodeEvolver enzyme engineering technology platform for the research, development and manufacture of novel enzymes for use by Merck in the chemical synthesis of therapeutic products owned or controlled by Merck (Merck Exclusive Field) and a non-exclusive worldwide license to use the CodeEvolver enzyme engineering technology platform to research, develop and manufacture novel enzymes for use by Merck in its internal research programs (Merck Non-Exclusive Field).
In September 2016, the company completed the full transfer of the engineering platform technology. In October 2018, the company entered into an amendment to the Merck CodeEvolver Agreement whereby the company amended certain licensing provisions and one exhibit. In January 2019, the company entered into an amendment to the Merck CodeEvolver Agreement whereby the company installed certain CodeEvolver enzyme engineering technology upgrades into Merck’s platform license installation. The company maintained those upgrades for a multi-year term that expired in January 2022.
Novartis
In May 2019, the company entered into a Platform Technology Transfer and License Agreement (the ‘Novartis CodeEvolver Agreement’) with Novartis. The Novartis CodeEvolver Agreement allows Novartis to use the company’s proprietary CodeEvolver enzyme engineering platform technology in the field of human healthcare.
Under the terms of the Novartis CodeEvolver Agreement, the company granted to Novartis a worldwide license to use certain patents, patent applications and know-how from its CodeEvolver enzyme engineering technology platform to research, develop and manufacture novel enzymes for use by or on behalf of Novartis as biocatalysts in the chemical synthesis of small molecule and bioconjugate APIs. The license is exclusive for the research, development and manufacture of novel enzymes for use by Novartis as biocatalysts in the chemical synthesis of API owned or controlled by Novartis (Novartis Exclusive Field) and non-exclusive license for the research, development and manufacture of novel enzymes for use by Novartis in the chemical synthesis of API not owned or controlled by Novartis or any third party (Novartis Non-Exclusive Field).
In July 2021, the company announced the completion of the technology transfer period during which the company transferred its proprietary CodeEvolver platform technology to Novartis (the ‘Technology Transfer Period’).
Intellectual Property
As of December 31, 2022, the company owned or controlled approximately 2,090 issued patents and pending patent applications in the United States and in various foreign jurisdictions, many of which are directed to its enabling technologies and specific methods and products that support its business in the pharmaceutical markets. The company’s patents and pending patent applications, if issued, have terms that expire between 2023 and approximately 2043. The company’s United States (U.S.) patents and pending patent applications directed to the CodeEvolver proprietary enabling technology platform developed internally by the company have terms that expire between 2029 and approximately 2034. It is possible that some U.S. patents and patent applications (if issued) may be entitled to patent term extensions and/or patent term adjustments, which would extend the protection beyond these expiration dates. It is also possible that some patents and patent applications (if issued) in other jurisdictions will be entitled to additional patent term. The company’s intellectual property rights also include patents, trademarks, copyrights, software and certain assumed contracts that the company acquired from Maxygen, Inc. (Maxygen) in October 2010, which are associated with directed evolution technology, known as the MolecularBreeding technology platform developed by Maxygen. The intellectual property rights and other related assets that the company acquired from Maxygen continue to be subject to existing exclusive and non-exclusive license rights granted by Maxygen to third parties. The company continues to file new patent applications, for which terms generally extend 20 years from the non-provisional filing date in the United States.
As of December 31, 2022, the company owned approximately 100 trademark registrations in the United States and foreign jurisdictions, as well as many common law trademarks. These include, but are not limited to: Codexis, Codex, CodeEvolver, Mosaic, Sage, Microcyp, MCYP, ProSAR, Unlock the Power of Proteins, the Codexis Protein Engineering Experts logo, Strategist, Continuity, Ameli, Forager, Analogene, Harvester, Atoms, Riptide, APS and a Codexis design mark (i.e., the stylized Codexis logo).
Government Regulation
The company’s biotherapeutic product candidates are subject to regulation by the FDA as biologics.
Research and Development
The company’s research and development expenses were $80.1 million in 2022.
Competition
Performance Enzyme
Pharmaceutical Manufacturing
The company competes with companies developing and marketing conventional catalysts, including Solvias AG, BASF, Johnson-Matthey and Takasago International Corporation.
Life Sciences
Several of the company’s competitors, such as ThermoFisher Scientific, Roche Diagnostics (a division of Roche Holding AG), New England Biolabs (NEB), and QIAGEN group offer a wide diversity of products across the life sciences market, including products that support multiple applications in RNA manufacturing and genomics. The company also competes with companies that are more focused on offering products and services for RNA manufacturing, such as Aldevron (a Danaher company) as well as companies focused on providing enzymes and services to genomic sequencing applications, such as Promega Corporation and Watchmaker Genomics.
History
Codexis, Inc. was founded in 2002. The company was incorporated in Delaware in 2002.

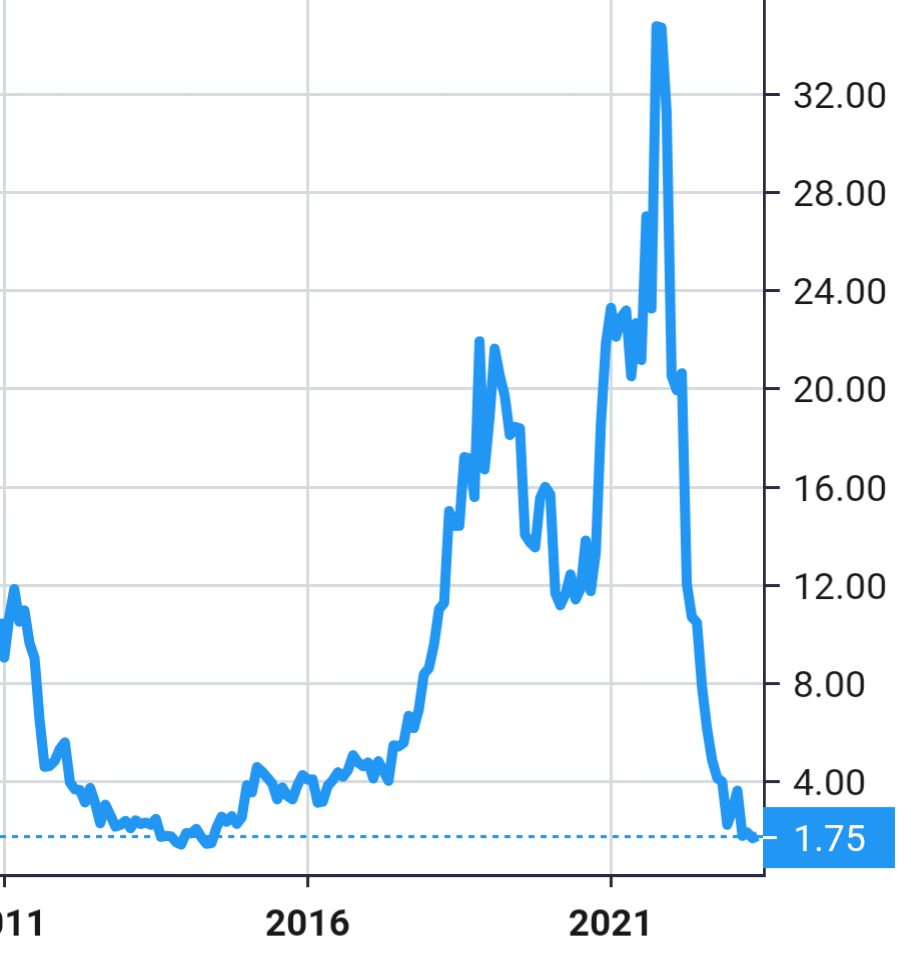
 Stock Value
Stock Value

