About CureVac N.V.
CureVac N.V. (CureVac), together with its subsidiaries, operates as an integrated biopharmaceutical company.
The company engages in developing a new class of transformative medicines based on messenger ribonucleic acid that has the potential to improve the lives of people. The company's technology platform is based on a targeted approach to optimize messenger ribonucleic acid (mRNA) constructs that encode functional proteins that either induce a desired immune response or replace defective or missing proteins using the cell's intrinsic translation machinery. The company's product portfolio includes clinical and preclinical candidates across multiple disease indications in prophylactic vaccines, oncology, and molecular therapy.
In prophylactic vaccines, the company is advancing its second-generation mRNA backbone against coronavirus (SARS-CoV-2) and a range of infectious diseases, including influenza in collaboration with GlaxoSmithKline Biologicals SA (GSK).
The collaboration on COVID-19 vaccine candidates with GSK was initiated in April 2021, and aims to research, develop, and manufacture mRNA vaccines targeting the original SARS-CoV-2 strain, as well as emerging variants.
CV2CoV was introduced as the first representative of the company's joint COVID-19 vaccine program based on its second-generation backbone. The vaccine candidate is a non-chemically modified mRNA, encoding the prefusion stabilized full-length spike protein of the original SARS-CoV-2 virus, formulated within Lipid Nanoparticles (LNPs). On June 30, 2021, the company published preclinical data in Nature Communications demonstrating full protection by CV2CoV and its first-generation vaccine candidate CVnCoV, from lethal infection caused by SARS-CoV-2 ancestral strain BavPat1 or the Beta variant (B.1.351) in a transgenic mouse model. On August 4, 2022, the company further published in the journal Vaccines data from a preclinical study in rats, showing that CV2CoV is able to induce high levels of antigen production in an in vitro setup, as well as strong and dose-dependent immune responses in vivo. In a subsequent Nature publication issued on November 18, 2021, the company further presented preclinical data investigating immune responses, as well as the protective efficacy of CV2CoV in comparison to CVnCoV, against SARS-CoV-2 challenge in non-human primates.
On April 21, 2022, the preclinical data for CV2CoV and the second-generation mRNA backbone was extended by a study conducted in collaboration with the Friedrich-Loeffler-Institute, comparing immune responses and protective efficacy of monovalent and bivalent mRNA vaccines encoding Beta and/or Delta variants, primarily in a transgenic mouse model and a Wistar rat model. On March 30, 2022, the company announced the start of a Phase 1 clinical trial with CV2CoV. The Phase 1 dose-escalation study is being conducted at clinical sites in the United States and evaluates the safety, reactogenicity and immunogenicity of a single booster dose of CV2CoV in the dose range of 2µg to 20µg.

Within the joint vaccine program with GSK, the company also extended its technology platform to chemically modified mRNA constructs to allow for data-driven selection of the best candidate. The company announced the start of a Phase 1 clinical trial with a chemically modified COVID-19 mRNA vaccine candidate based on its second-generation backbone, CV0501, on August 18, 2022. The study is being conducted at clinical sites in the United States, Australia, and the Philippines and evaluates the safety, reactogenicity, and immunogenicity of a single booster dose of CV0501 in the dose range of 3µg to 200µg.
On January 6, 2023, the company announced that the second-generation mRNA backbone using modified mRNA was selected as the preferred technology for further clinical development in the COVID-19 program. In January and April 2023, it also announced positive preliminary data from the CV0501 Phase 1 trial. A Phase 2 clinical study, expected to start later in 2023, will assess monovalent and/or bivalent vaccine candidates designed to target clinically relevant variants. A pivotal Phase 3 trial may be initiated in 2024, contingent on discussion with regulatory authorities.
Influenza was disclosed as the first indication from the initial collaboration the company started with GSK in July 2020, which focuses on the development of new products for different targets in the field of infectious diseases.
The first non-COVID-19 vaccine candidate within the broader infectious disease program applying the company's second-generation backbone it tested in collaboration with GSK is the influenza candidate, CVSQIV, a differentiated multivalent vaccine candidate featuring multiple non-chemically modified mRNA constructs to induce immune responses against relevant targets of four different influenza strains. On February 10, 2022, the company announced the start of a Phase 1 dose-escalation study in Panama evaluating the safety, reactogenicity and immunogenicity of CVSQIV in the dose range of 3µg to 28µg. Preliminary safety data reported on April 28, 2022, showed a benign reactogenicity profile across the tested dose groups.
In line with the mRNA development strategy to also test chemically modified mRNA and similar to the setup of the COVID-19 vaccine program, CureVac and GSK announced the start of a Phase 1 dose-escalation study with a chemically modified influenza vaccine candidate, Flu SV mRNA, on August 18, 2022. The Phase 1 dose-escalation study is being conducted in Canada, Spain, and Belgium to evaluate the safety, reactogenicity and immunogenicity of FLU SV mRNA.
On January 6, 2023, the second-generation mRNA backbone using modified mRNA was selected as the preferred technology for further clinical development in the seasonal flu vaccine program. In the Phase 1 study of the monovalent Flu-SV-mRNA, expressing an H1N1 hemagglutinin antigen (subtype of influenza A), five doses ranging from 2 to 54 microgram with up to 25 subjects per dose cohort were evaluated in younger adults (age 18-45). In younger adults, adjusted geometric mean hemagglutinin inhibition antibody titers elicited by Flu-SV-mRNA increased up to approximately 3.3 times those elicited by the licensed flu vaccine comparator in younger adults. In older adults, adjusted geometric mean hemagglutinin inhibition antibody titers elicited by Flu-SV-mRNA were approximately 2.3 times those elicited by the licensed flu vaccine comparator. In the same age group, the percentage of subjects achieving seroconversion was 89.7% for Flu-SV-mRNA and 56.2% for the licensed flu vaccine comparator.
The vaccine candidate for future clinical development is expected to target all four strains recommended by the WHO for influenza vaccines. A Phase 1/2 study for multivalent vaccine candidates is expected to start in the second quarter of 2023.
In March 2022, the company established the CureVac RNA Printer GmbH as a wholly owned CureVac company to advance The RNA Printer. The new entity is designed as a platform and services company, providing a dedicated operational environment to further develop and establish The RNA Printer as a manufacturing end-to-end solution.
On October 12, 2021, the company announced the strategic decision to withdraw its first-generation COVID-19 vaccine candidate, CVnCoV, from the approval process with the European Medicines Agency (EMA), and to focus its COVID-19 vaccine program on the development of second-generation mRNA vaccine candidates in collaboration with GSK. The rolling submission with the EMA was originally initiated in February 2021 to assess CVnCoV's compliance with standards for vaccine efficacy, safety, and pharmaceutical quality as a prerequisite for a formal market authorization application. Later in 2021, the EMA informed the company that it would not start reviewing the provided CVnCoV data packages before 2022. As a result, it estimated that the earliest possible approval of CVnCoV would come in the second quarter of 2022. Consequently, CVnCoV was also withdrawn from a rolling submission with Swissmedic, Switzerland's authority responsible for the authorization and supervision of therapeutic products, initiated in April 2021, to review the safety, efficacy and pharmaceutical quality of CVnCoV as a prerequisite for market authorization.
All clinical studies with first-generation candidate, CVnCoV, have completed the scheduled safety follow-up times for all trial participants as per the respective trial protocols. These include a Phase 1 study in Germany (initiated in June 2020), a Phase 2a study in Peru and Panama (initiated in September 2020), the Phase 2b/3 (HERALD) study in Europe and Latin America (initiated in December 2020), a Phase 3 study in healthcare workers in Germany (initiated in December 2020), and a Phase 3 study in participants with comorbidities in Belgium (initiated in April 2021).
Data analyses of the Phase 2b/3 (HERALD) study and Phase 3 study in healthcare workers in Germany have been finalized. Primary data of the Phase 2b/3 (HERALD) trial was published in The Lancet Infectious Diseases on November 23, 2021. Data of an interim analysis of the Phase 1 trial in Germany was published in Wiener klinische Wochenschrift on August 10, 2021. Safety and immunogenicity data of the Phase 2a clinical trial in Peru and Panama was published in Vaccine: X on July 1, 2022. Neutralizing antibody data against the ancestral strain and the beta variant after a third dose of CVnCoV in the same trial were published in Vaccines on March 25, 2022.
To assess the benefit of booster vaccinations, CVnCoV was also included in the Cov-Boost trial sponsored by the University of Southampton, the U.K. The Cov-Boost trial started in June 2021 across 18 sites in the United Kingdom and dosed overall 2,878 participants with a third dose vaccine. Initial results from the Cov-Boost trial were published in The Lancet on December 2, 2021.
Beyond the GSK COVID-19 and general infectious disease collaboration, the company's next advanced prophylactic vaccine program, CV7202, is being developed for prophylactic vaccination against rabies. CV7202 is an mRNA based on its first-generation backbone that encodes the rabies virus glycoprotein, RABV-G, formulated with Lipid Nanoparticles. Safety, reactogenicity, and immunogenicity of CV7202 was investigated in a Phase 1 clinical trial that has completed the scheduled follow-up time for all trial participants as per trial protocol. In January 2021, the company published data from its Phase 1 trial of CV7202 in Vaccine.
The company is evaluating targeted expansions of its unique mRNA approaches for the development of cancer vaccines.
The company's clinical oncology candidate, CV8102, is a complex of single-stranded non-coding RNA, which has been optimized to maximize activation of cellular receptors that normally detect viral pathogens entering the cells (such as toll-like receptor 7, or TLR7, toll-like receptor 8, or TLR8, and retinoic acid inducible gene I, or RIG-I pathways), mimicking a viral infection of the tumor.
In February 2021, the company initiated an expansion of its Phase 1 study to confirm the safety, tolerability, and efficacy of CV8102 at a 600 microgram dose in 40 patients with advanced melanoma. On November 11, 2022, the company presented preliminary data from the expansion study. As of August 30, 2022, preliminary efficacy was observed in the cohort of 30 patients treated in combination with anti-PD-1 antibodies, 40% of whom were pretreated with anti-CTLA-4 antibodies. In this anti-PD-1 combination cohort, five out of 30 patients (17%) experienced a partial response according to RECIST 1.1.
Final study results are expected to be submitted for publication in a peer reviewed journal in the first half of 2023. A scientific paper assessing the mode of action and efficacy of CV8102 for local immunotherapy in preclinical models was published on November 2, 2022, in Cancer Immunology, Immunotherapy.
In molecular therapies, the company published preclinical mouse data in liver fibrosis in the Journal of Hepatology in August 2021. Progression of liver fibrosis is associated with the gradual decrease of hepatocyte nuclear factor 4 alpha (HNF4 alpha) an important regulator and key factor in liver metabolism. In the published study, four independent mouse models of the disease were treated with mRNA encoding HNF4A. The treatment was able to restore HNF4A levels and thereby significantly reduced liver injury. The study was conducted in collaboration with the REBIRTH-Research Center for Translational Regenerative Medicine and Department of Gastroenterology, Hepatology and Endocrinology at the Hannover Medical School, Hannover (Germany). It provides the first preclinical data demonstrating the therapeutic applicability of mRNA encoded HNF4A in the treatment of liver fibrosis and cirrhosis.
The company is advancing undisclosed programs in preclinical studies across eye disorders, as well as delivering therapeutic antibodies.
Product Portfolio
The company's differentiated mRNA technology platform is designed to address a broad range of diseases across multiple therapeutic areas. Given the strengths of its platform, the broad potential of mRNA-based medicines and its rational approach to disease selection, it has chosen to leverage its platform to initially focus on advancing its product candidates in the areas of prophylactic vaccines, oncology, and molecular therapy.
For the immune active side of the company's technology, it focuses on RNA or mRNA-based medicines in prophylactic vaccines and oncology. For the immune silent side of its technology, it has expanded its preclinical product portfolio to include mRNA therapies based on the expression of therapeutic proteins (including ocular, liver, and lung applications).
The company's lead programs include:
The company's second-generation construct CV0501 against SARS-CoV-2 is a monovalent construct applying modified mRNA developed in collaboration with GSK. It initiated a Phase 1 study in August 2022, which provided positive preliminary results in January 2023.
The company's second-generation vaccine candidate Flu SV mRNA against influenza is a monovalent construct applying modified mRNA developed in collaboration with GSK. It initiated a Phase 1 study in August 2022, which provided positive preliminary results in January 2023.
In oncology, the company focuses on building a meaningful portfolio of novel cancer vaccine candidates based on the differentiated antigen discovery technologies it acquired with Frame Cancer Therapeutics and its partnership with myNEO. Within this portfolio, it follows two approaches. The first approach assesses tumor antigens shared by different cancer patients for the development of off-the-shelf cancer vaccines. The second approach is tailored to the individual tumor setup of a patient for personalized therapy. A proof-of-principle study, assessing an mRNA construct encoding multiple epitopes from eight tumor associated antigens in patients with surgically resected Glioblastoma Multiforme is planned to start in the second quarter of 2023.
The company's key partnered programs include:
The company has partnered with GSK for the development of COVID-19 vaccine candidates based on its second-generation mRNA backbone, including CV0501 and CV2CoV, and vaccine candidates based on its second-generation mRNA backbone against other infectious diseases, including Flu SV mRNA and CVSQIV against influenza.
The company has partnered with the immunotherapy company myNEO to identify specific antigens on the surface of tumors for the development of novel mRNA-based cancer vaccine candidates. The partnership leverages myNEO's biological datasets and integrated machine learning and bioinformatics platform to identify and validate specific tumor antigens predicted to elicit a strong immune response.
The company has partnered with CRISPR Therapeutics for the development of novel Cas9 mRNA constructs for use in gene editing therapeutics, with improved properties, such as increased potency, decreased duration of expression, and reduced potential for immunogenicity. CRISPR Therapeutics has an exclusive license to the improved constructs in three of their in vivo gene editing programs.
The company has a broad strategic partnership with Genmab to leverage its mRNA technology platform to develop up to four mRNA-based novel therapeutic antibodies. This represents the first publicly announced strategic partnership focused on differentiated mRNA-based antibodies.
The company has received grants from the Bill & Melinda Gates Foundation to develop prophylactic vaccines designed to prevent picornaviruses, influenza, malaria, and rotavirus.
The company also has several academic collaborations, including with SERI for target discovery research in mRNA-based eye therapy.
The company's product candidates consist of two major components: the protein-coding mRNA and a delivery vehicle. Once it has established delivery capability to a target tissue, it can design new product candidates that vary only in the mRNA component, which it expects will allow for rapid target and development candidate identification.
Prophylactic Vaccines
The company's approach to the development of potential prophylactic vaccines focuses on:
CV0501 for SARS-CoV-2: The company's second-generation mRNA vaccine candidate against SARS-CoV-2 is a monovalent construct, applying modified mRNA developed in collaboration with GSK. A Phase 1 study was initiated in August 2022. It reported positive preliminary data in early 2023. A Phase 2 clinical study, expected to start later in 2023, will assess monovalent and/or bivalent vaccine candidates designed to target clinically relevant variants.
Flu SV mRNA for influenza: The company's second-generation mRNA vaccine candidate against influenza is a monovalent construct, applying modified mRNA developed in collaboration with GSK. A Phase 1 study was initiated in August 2022. It reported positive preliminary data in early 2023. The vaccine candidate for future clinical development is expected to target all four strains recommended by the World Health Organization (WHO) for influenza vaccines. A Phase 1/2 study for multivalent vaccine candidates is expected to start in the second quarter of 2023.
Oncology
mRNA is a versatile platform for cancer vaccine development allowing to encode a wide range of antigens from full length tumor associated antigens to neoepitopes. The company is taking multiple approaches in oncology to induce tumor-specific immune responses in patients:
Novel Cancer Vaccines: The company focuses on building a meaningful portfolio of novel cancer vaccine candidates based on differentiated antigen discovery technologies and bioinformatics to target antigens that are overexpressed in tumor tissues with no or little expression on healthy tissues, using its Lipid Nanoparticles (LNP) formulations. Within this strategy, the company follows two approaches. The first approach assesses tumor antigens shared by different cancer patients for the development of off-the-shelf cancer vaccines. The second approach is tailored to the individual tumor setup of a patient for personalized therapy.
The company has demonstrated in a preclinical model that an optimized LNP formulated mRNA vaccine, encoding a TAA, that is also a self-antigen, can induce cellular and anti-tumoral immune responses and single-agent therapeutic activity. These immune responses led to single-agent therapeutic effect in the B16F10 tumor model that does not respond to anti-PD-1 antibodies alone. The therapeutic effect of the vaccine was further enhanced by concomitant systemic anti-PD-1 antibody treatment.
Intratumoral Therapy: Intratumoral injection of immunostimulating agents into tumors is an alternative to classic vaccination to induce a therapeutic immune response. High concentration of such agents can be achieved by local administration in the tumor tissue with little systemic side effects. Intratumoral immunotherapy activates antigen-presenting cells in the tumor environment and draining lymph nodes to present a broad panel of antigens expressed by the tumor to T and B cells and induce a systemic immune response against the injected tumor as well as non-injected metastatic lesions (abscopal effect).
The company's has collaborations that focuses on:
Therapeutic Antibodies: The company is also developing mRNA therapies to produce antibodies systemically using the liver as a bioreactor for subsequent secretion and systemic distribution of the antibodies to primary organs affected by a disease. Its collaboration with Genmab, a global leader in antibody discovery and design, will allow the company to work with novel antibodies produced using its mRNA technology. This partnership represents the first-ever publicly disclosed mRNA antibody-focused deal and will allow it to optimize and manufacture mRNA-encoded antibodies for Genmab.
Liver Diseases: The company is working on mRNA therapies targeted for the liver, including designing and producing intracellular targets, such as transcription factors. Its collaboration with the Hannover Medical School aims to design treatments for liver diseases, such as NAFLD, NASH, and liver cirrhosis, with the potential to treat or reverse liver fibrosis.
Eye Diseases: The company is investigating development of mRNA-based treatments for undisclosed ophthalmic indications. It has a collaboration with The Schepens Eye Research Institute, Inc. (SERI) for its discovery efforts.
Key Pipeline Candidates
CV8102
CV8102 is the first compound the company is developing for the treatment of various solid tumors using an intratumoral approach. CV8102 is based on a complex of single stranded non-coding RNA with a polymeric peptide that binds and coats the RNA, protecting it from rapid degradation while also helping to stimulate the immune system.
CV8102 was shown to activate cellular receptors that normally detect viral pathogens entering the cells (such as TLR7, TLR8, and RIG-I pathways). By mimicking a viral infection at the injection site, CV8102 is designed to induce an inflammation that can activate the immune system to reject the tumor. CV8102 was initially developed as a vaccine adjuvant and was shown to enhance the induction of multifunctional CD8 T cell responses and therapeutic activity of peptide vaccines against cancer in preclinical models.
CV8102 is in a Phase 1 clinical trial for the intratumoral treatment of four types of solid tumors-cutaneous melanoma (cMEL), adenoidcystic carcinoma (ACC), and squamous cell carcinoma of skin (SCC), as well as squamous cell carcinoma of head and neck (HNSCC). Enrollment was completed in October 2021, and 98 patients were enrolled, 58 patients in the dose escalation part and 40 patients in a dose expansion part in Melanoma.
The company initiated a Phase 1 clinical trial of CV8102 for the treatment of various solid tumors in 2017. The Phase 1 clinical trial is evaluating intratumoral administration of CV8102 in patients with advanced melanoma, squamous cell carcinoma of the skin, squamous cell carcinoma of the head and neck, or adenoid cystic carcinoma. Patients receive CV8102 as single-agent or in combination with anti-PD-1 therapy. Patients with advanced inoperable melanoma, cutaneous or head and neck squamous cell or adenoid cystic carcinoma are eligible for single-agent CV8102, and patients with advanced inoperable melanoma and head and neck squamous cell carcinoma indicated for anti-PD-1 therapy or who did not respond or slowly progressed on anti-PD-1 therapy are eligible for the combination. CV8102 is administered for up to eight intratumoral injections into a single accessible tumor lesion over a 12-week period.
CV7202
CV7202 is the company's rabies vaccine candidate encoding the rabies virus glycoprotein, RABV-G protein formulated with LNPs. RABV-G is one of only five proteins encoded by the rabies virus. As a dominant part of the virus surface and its role in virus entry into the host cell, RABV-G is the only target of virus-neutralizing antibodies conferring protection against challenge.
A Phase 1 clinical trial for CV7202 initiated in the fourth quarter of 2018 has been completed with all follow-up times as per trial protocol. Data of the Phase 1 trial was published in Vaccine on January 22, 2021.
The Phase 1 clinical trial for CV7202, initiated in the fourth quarter of 2018, was conducted as a non-randomized, open-label, controlled, dose-escalation, multi-center Phase 1 study evaluating safety, reactogenicity and immunogenicity of different dosages of CV7202 administered as intramuscular injections in healthy adults 18 to 40 years of age in one- or two-dose regimens.
CV-SSIV
CV-SSIV contains a mixture of antigens derived from hemagglutinin (HA), and neuraminidase (NA), constructs, all from seasonal strains recommended by the WHO, targeting both Influenza A and B strains.
The company is also developing a Supra Seasonal Influenza Vaccine (SSIV).
Other Prophylactic Vaccines for Infectious Diseases
In partnership with the Bill & Melinda Gates Foundation, the company is developing prophylactic vaccines for prevention of other infectious diseases associated with high mortality in the developing world, including malaria and rotavirus. Preclinical studies are ongoing, with encouraging results, which could lead to the decision for further clinical development of the candidate vaccines.
Furthermore, the company is collaborating on several vaccine projects with Coalition for Epidemic Preparedness Innovations (CEPI), a public-private initiative to strengthen the vaccine research. This focuses on the development of the mRNA Printer, a mobile, automated production unit for rapid mRNA supply. This innovative platform is being designed to provide a rapid supply of LNP-formulated mRNA vaccine candidates that can target known pathogens (including Lassa fever, yellow fever and SARS-CoV-2) and prepare for rapid response to new and previously unknown pathogens.
Strategy
The key components of the company's strategy are to continue to invest in its proprietary technology platform to be the leading mRNA platform company; utilize a rational disease selection approach to minimize clinical and commercial risk for the company's programs and broader platform; rapidly advance the company's lead product candidates through clinical development and regulatory approval; continue to invest in its manufacturing capabilities across all manufacturing steps from starting material to formulation to further add scale and flexibility for potential commercialization; selectively seek strategic partners to develop and commercialize product candidates in certain therapeutic areas and geographies; seek strategic acquisitions or in-licenses of technology or assets that are complementary to its programs and technology platform; and strengthen and expand its intellectual property portfolio to protect its scientific and technical know-how.
Collaborations
In July 2020, the company entered into a Collaboration and License Agreement with GSK, which it refers to as the 2020 GSK Agreement, pursuant to which it is collaborating with GSK to research, develop, and commercialize prophylactic and therapeutic non-replicating mRNA-based vaccines and antibodies targeting infectious disease pathogens.
In April 2021, the company entered into a collaboration agreement with GSK, which it refers to as the GSK COVID Agreement, pursuant to which it is collaborating with GSK to research, develop, and manufacture next-generation mRNA vaccines targeting the original SARS-CoV-2 strain, as well as emerging variants, including multivalent and monovalent approaches, such as its second-generation COVID-19 vaccine candidate, CV2CoV.
In December 2019, the company entered into a Collaboration and License Agreement with Genmab, which it refers to as the Genmab Agreement, to research and develop up to four potential differentiated mRNA-based antibody products, to be selected by Genmab, based on the combination of its proprietary RNAntibody technology with Genmab's proprietary antibody technology for the treatment of human diseases. The Genmab Agreement was amended in July 2020, December 2020, and June 2021.
In January 2018, the company entered into a Development and Option Agreement with Arcturus, which it refers to as the Arcturus Agreement, pursuant to which Arcturus granted it the right to reserve a certain number of targets and an irrevocable offer to obtain a license to a certain number of such reserved targets to develop, manufacture, and commercialize products containing Arcturus's LNP technology (LMD technology) and mRNA constructs intended to express such targets. The Arcturus Agreement was amended in May 2018, September 2018, and July 2019. As of December 31, 2022, the company had not accepted the offer with respect to any targets.
In April 2016, the company entered into a Development and Option Agreement with Acuitas, which as amended it refers to as the Acuitas Agreement, pursuant to which Acuitas granted it the right to reserve a certain number of vaccine and other targets and an option to obtain a license to a certain number of such reserved targets to develop, manufacture and commercialize products containing Acuitas's LNP technology and mRNA constructs intended to express such targets. Under the Acuitas License Agreements, Acuitas grants the company a non-exclusive, non-transferable, sublicensable (subject to certain conditions) worldwide license under Acuitas's LNP technology to develop, manufacture, and commercialize licensed products directed to the optioned targets.
In November 2017, the company entered into a Development and License Agreement with CRISPR Therapeutics, which as amended by an amendment entered into in June 2020, it refers to as the CRISPR Therapeutics Agreement, pursuant to which it will develop novel Cas9 mRNA constructs for use in gene editing therapeutics. Under the terms of the CRISPR Therapeutics Agreement, the company granted CRISPR Therapeutics a worldwide, exclusive (even to it), sublicensable (subject to certain conditions) license under certain intellectual property rights that are reasonably necessary or useful to develop, manufacture, or commercialize products comprising Cas9 mRNA constructs, and under any patents controlled by it that arise from inventions discovered under the CRISPR Therapeutics Agreement to develop, manufacture, and commercialize three of CRISPR Therapeutics' in vivo gene-editing programs for certain diseases. CRISPR Therapeutics granted the company an exclusive (even as to CRISPR Therapeutics), worldwide, cost-free sublicense to manufacture products comprising Cas9 mRNA constructs for CRISPR Therapeutics.
In November 2015, the company entered into a development and intellectual property agreement with Tesla Automation, which it refers to as the Tesla Automation Agreement, pursuant to which Tesla Automation agreed to design, develop, and manufacture certain automated manufacturing machines on the company's behalf.
Sponsored Collaboration Agreements
In March 2019, the company entered into a sponsored research agreement, as amended in April 2020, July 2021, September 2021, August 2022, and January 2023 (as amended, the Schepens Agreement), with The Schepens Eye Research Institute, Inc. (SERI) and Massachusetts Eye and Ear Infirmary (MEEI), pursuant to which SERI and MEEI agreed to perform certain research activities for mRNA-based eye therapy candidates. Under the Schepens Agreement, SERI and MEEI granted the company an exclusive option to initiate negotiations for an exclusive or non-exclusive license to SERI's interest in any inventions developed under the Schepens Agreement. SERI and MEEI additionally granted it an exclusive option to negotiate an exclusive license to certain background intellectual property.
Intellectual Property
The company has built an intellectual property portfolio in the United States, Europe, and other major geographies. As of April 3, 2023, it owned approximately 803 issued patents worldwide, including 102 issued U.S. patents, 50 issued European patents (which have been validated in various European countries resulting in a total of approximately 518 national patents in European countries), and 183 issued patents in other foreign countries, 122 pending U.S. patent applications, 85 pending European patent applications, 262 pending patent applications in other foreign countries and 17 pending PCT patent applications.
Patents
As of April 3, 2023, the company owned approximately 102 issued U.S. patents, 122 pending U.S. patent applications, 233 issued foreign patents (including 50 European patents, which have been validated in various European countries resulting in a total of approximately 518 national patents in European countries), 347 pending foreign patent applications (including 85 pending European patent applications) and 17 pending Patent Cooperation Treaty, or PCT, patent applications, including several patent families that are jointly owned with third parties. These patents include claims relating to the company's RNAoptimizer technology platform, CV8102, CV7202, CVSQIV, its COVID-19 vaccine candidates, and its proprietary LNP technology.
RNAoptimizer
As of April 3, 2023, the company owned 26 issued U.S. patents, 18 pending U.S. patent applications, 106 issued foreign patents, including in Europe, Canada, China, India, Japan, the Republic of Korea, Singapore, Russia, Mexico, and Australia, and 103 pending foreign patent applications and five PCT patent applications relating to its RNAoptimizer technology, including patents and patent applications relating to ORF optimization, UTR optimization, protein optimization and formulation. The issued patents are expected to expire between 2025 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending patent applications would be expected to expire between 2023 and 2044, excluding any additional term for patent term adjustments or patent term extensions.
CV8102
As of April 3, 2023, the company owned four issued U.S. patents, five pending U.S. patent applications, 26 issued foreign patents, including in Europe, Brazil, Canada, China, India, Japan, the Republic of Korea, Singapore, Taiwan, Russia, Mexico, and Australia; and 19 pending foreign patent applications relating to its CV8102 product candidate. The issued patents are expected to expire between 2028 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending applications would be expected to expire between 2029 and 2037, excluding any additional term for patent term adjustments or patent term extensions.
CV7202
As of April 3, 2023, the company owned eight issued U.S. patents, five pending U.S. patent applications, 25 issued foreign patents, including in Europe, Canada, China, India, Japan, the Republic of Korea, Singapore, Russia, Mexico, and Australia; and 23 pending foreign patent applications relating to its CV7202 product candidate. The issued patents are expected to expire between 2025 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending patent applications would be expected to expire between 2023 and 2037, excluding any additional term for patent term adjustments or patent term extensions.
CV-SQIV
As of April 3, 2023, the company owned 10 issued U.S. patents, 13 pending U.S. patent applications, 44 issued foreign patents, including in Europe, Canada, China, India, Japan, the Republic of Korea, Singapore, Russia, Mexico, and Australia; and 56 pending foreign patent applications relating to its CVSQIV product candidate. The issued patents are expected to expire between 2025 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending patent applications would be expected to expire between 2023 and 2043, excluding any additional term for patent term adjustments or patent term extensions.
COVID-19 Vaccine Candidates
As of April 3, 2023, the company owned 13 issued U.S. patents, 15 pending U.S. patent applications, 43 issued foreign patents, including in Europe, Canada, China, India, Japan, the Republic of Korea, Singapore, Russia, Mexico, and Australia; 71 pending foreign patent applications and one PCT patent application relating to the company's COVID-19 product candidates, CVnCoV and CV2CoV. The issued patents are expected to expire between 2025 and 2041, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending patent applications would be expected to expire between 2023 and 2043, excluding any additional term for patent term adjustments or patent term extensions.
Trademarks
As of April 3, 2023, the company owned trademark registrations or registration applications for CureVac, and the CureVac logo in the United States and in certain foreign jurisdictions, including Europe.
Research and Development
The company's research and development costs were €62.6 million for the year ended December 31, 2022.
History
CureVac N.V. was founded in 2000. The company was incorporated pursuant to the laws of the Netherlands in 2020.
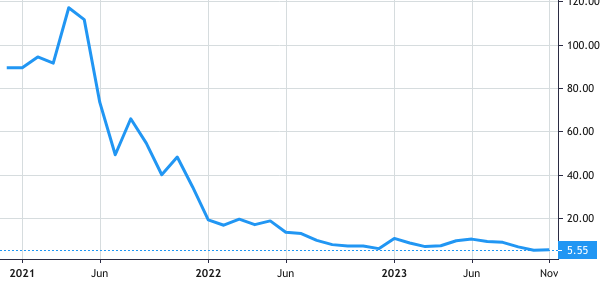
 Stock Value
Stock Value

