About Exact Sciences
Exact Sciences Corporation and its subsidiaries (Exact) operate as a global, advanced cancer diagnostics company.
The company has developed some of the most impactful tests in cancer screening and diagnostics; and is working to develop additional tests, with the goal of bringing new, innovative cancer tests to patients throughout the world.
The company’s revenues are primarily generated by the company’s laboratory testing services from the company’s Cologuard colorectal cancer screening test and the company’s Oncotype DX cancer diagnostic tests and services.
Products and Services
With a leading portfolio of products for earlier cancer detection and treatment guidance, the company provides patients with earlier, smarter answers. The company’s products and services focus on screening and precision oncology tests.
Screening Tests

Cologuard Test
The company’s flagship screening product, the Cologuard test, is a patient-friendly, non-invasive, stool-based DNA (‘sDNA’) screening test that utilizes a multi-target approach to detect DNA and hemoglobin biomarkers associated with colorectal cancer and pre-cancer. Eleven biomarkers are targeted that have been shown to be strongly associated with colorectal cancer and pre-cancer. Methylation, mutation, and hemoglobin results are combined in the laboratory analysis through a proprietary algorithm to provide a single positive or negative reportable result.
The large, underserved population of unscreened and inadequately screened patients represents a significant opportunity for the company’s Cologuard test.
Upon approval by the FDA in August 2014, the company’s Cologuard test became the first and only FDA-approved sDNA non-invasive colorectal cancer screening test. In September 2019, the FDA expanded its indication to include average-risk individuals ages 45-49. The company’s Cologuard test is now indicated for average risk adults 45 years of age and older.
Clinical Genetic Testing
The company provides more than 5,000 predefined genetic tests for nearly all clinically relevant genes, additional custom panels, and comprehensive germline, whole exome (‘PGxome’), and whole genome (‘PGnome’) sequencing tests.
The company’s hereditary cancer test, Riskguard, helps people understand their inherited risk of cancer, arming them with critical information to make better treatment decisions.
Precision Oncology Tests
The company’s precision oncology portfolio delivers actionable genomic insights to inform prognosis and cancer treatment after a diagnosis. The company enables patients to take a more active role in their cancer care and make it easy for providers to order tests, interpret results, and personalize medicine by applying real-world evidence and guideline recommendations.
Oncotype DX Breast Recurrence Score Test
The company’s Oncotype DX Breast Recurrence Score test has been demonstrated to identify patients who are most likely to benefit from chemotherapy, as well as those who may receive no clinical benefit from chemotherapy.
The Oncotype DX Breast Recurrence Score test examines the activity of 21 genes in a patient’s breast tumor tissue to provide personalized information for tailoring treatment based on the biology of the patient’s individual disease. The test is supported by multiple rigorous clinical validation studies, including the landmark TAILORx and RxPONDER studies, confirming the test’s ability to predict the likelihood of chemotherapy benefit, as well as the chance of cancer recurrence in certain common types of early-stage breast cancer.
As the only test proven to predict both the likelihood of chemotherapy benefit and cancer recurrence, the Oncotype DX Breast Recurrence Score test is globally recognized as standard of care and included in all major breast cancer treatment guidelines.
Oncotype DX Breast DCIS Score Test
The company’s Oncotype DX Breast DCIS Score test provides ductal carcinoma in situ (‘DCIS’) patients an individualized prediction of the 10-year risk of local recurrence (DCIS or invasive carcinoma), represented by a DCIS Score result. This test helps guide treatment decision-making in women with DCIS treated by local excision, with or without tamoxifen. Development of the company’s Oncotype DX Breast DCIS Score test was based on published results for the Oncotype DX Breast Recurrence Score test that showed similarity in the expression profiles of genes between DCIS and invasive breast cancer when both are present within the same patient tumor.
Oncotype DX Colon Recurrence Score Test
The company’s Oncotype DX Colon Recurrence Score test is a multi-gene test for predicting recurrence risk in patients with stage II and stage III A/B colon cancer to enable an individualized approach to treatment planning. By evaluating specific genes within a patient’s colon tumor, the test can determine the likelihood that the cancer cells will spread and cause the disease to return after surgery. Based on this information, healthcare providers and patients can make more informed treatment decisions. The Oncotype DX Colon Recurrence Score test is supported by three rigorous clinical validation studies confirming the test’s ability to provide additional and independent value beyond the used measures for determining colon cancer recurrence risk.
OncoExTra Test
In April 2021, the company began performing and selling the OncoExTra test, previously known as GEM ExTra, as a result of the company’s acquisition of Ashion Analytics, LLC (‘Ashion’). The OncoExTra test applies comprehensive tumor profiling, utilizing whole exome and whole transcriptome sequencing, to aid in therapy selection for patients with advanced, metastatic, refractory, relapsed, or recurrent cancer. With an extensive panel of approximately 20,000 genes and 169 introns, the OncoExTra test is one of the most comprehensive genomic (DNA) and transcriptomic (RNA) panels available today.
COVID-19 Testing Business
The company discontinued its COVID-19 testing operations in the second quarter of 2023. From March 2020 through June 2023, the company partnered with various customers, including the State of Wisconsin Department of Health Services, to administer testing. Customers were responsible for employing trained personnel to collect specimens. Specimens were sent to the company’s laboratory in Madison, Wisconsin, where the company ran the assay in the company’s laboratories and provided test results to ordering providers.
Pipeline Research and Development
The company’s research and development efforts are focused on developing new products and enhancing existing products to address unmet cancer needs and expand the clinical utility and addressable patient populations for the company’s existing tests. The company is focused on enhancing its Cologuard test’s performance characteristics and developing blood and other liquid-based (‘liquid biopsy’) tests. These development efforts may lead to a variety of new products, including risk assessment, screening and prevention, early disease diagnosis, adjuvant and/or neoadjuvant disease treatment, metastatic disease treatment selection, and patient monitoring.
Through the company’s collaboration with the Mayo Foundation for Medical Education and Research (‘Mayo’), the company has successfully performed validation studies involving multiple types of cancer using tissue, blood, and other sample types. In September 2020, Mayo agreed to make available certain personnel to provide the company research and development assistance through January 2025. Through recent business development activities, the company has also acquired exclusive access to technologies developed by The Johns Hopkins University (‘JHU’), Broad Institute, Inc. (‘Broad Institute’), Oxford University, and the Ludwig Institute for Cancer Research.
The company is focusing its research and development efforts on three main areas:
Colorectal Cancer Screening. The company is seeking to improve its Cologuard test’s performance characteristics, focusing on reducing the false positive rate of the test. In June 2023, the company and Mayo presented data from the prospective BLUE-C study, the FDA registrational trial for the company’s next-generation Cologuard test, showing overall sensitivity of 94% for colorectal cancer at specificity of 91%. The company submitted its next-generation Cologuard test for FDA approval in December 2023. The company is also working to develop a blood-based screening test for colorectal cancer as a second-line option for people who haven’t been screened with more accurate methods.
MCED Test Development. The company is seeking to develop a MCED test, which will be branded as Cancerguard to help detect many different types of cancer from a single blood draw. In September 2022, the company presented data at the European Society for Medical Oncology (‘ESMO’) Congress from a biomarker validation study, which demonstrated the ability to detect cancer signals from 15 organ sites with a mean sensitivity of 61% and mean specificity of 98.2%. The multi-biomarker approach detected stage I and stage II cancers with a combined sensitivity of 38.7%. In June 2023, the company announced a collaboration with Baylor Scott & White to utilize its Cancerguard test within a subset of their clinics to generate real-world experience and evidence of the company’s MCED approach. A larger case-control study is underway to further validate the results shared at ESMO and to determine the final design of the Cancerguard test. In the future, the company plans to begin recruiting patients for the FDA registrational Study of All comeRs (‘SOAR’) trial, which the company expects to be the largest prospective, interventional MCED trial ever conducted in the U.S.
MRD Test Development. The company plans to offer its Oncodetect test, a tumor-informed MRD test to help detect small amounts of tumor DNA that may remain in patients’ blood after they have undergone initial cancer treatment. This test will help patients and oncologists understand the success of initial treatment, guide further treatment, and monitor for cancer recurrence. The company’s intention is to support all patients in MRD and recurrence monitoring, whether there is access to tumor tissue to inform patient-specific biomarker targets or no access to tissue and a predefined biomarker panel is used. The company recently completed analytical validation of its tumor informed platform utilizing colorectal cancer samples and is conducting a clinical validation study for Medicare submission. In June 2023, the company entered into a sponsored research agreement and exclusive license agreement with Broad Institute to utilize their Minor Allele Enriched Sequencing Through Recognition Oligonucleotides (‘MAESTRO’) diagnostic testing technology to further the company’s ability to develop and launch impactful MRD tests.
Commercial Operations
The company has one core commercial function with specific teams focused on screening, precision oncology, and international markets.
Cologuard Test Commercial Operations
The company promotes its Cologuard test through a region and market-based model consisted of the company’s health systems, primary care, market development, and inside sales team members.
The company’s sales team actively engages with healthcare providers and their staff to emphasize the need for colorectal cancer screening, educate them on the value of the company’s Cologuard test, and facilitate their ability to order the test. The company focuses on specific healthcare providers based on a combination of Cologuard order history and ordering potential data. The company also focuses on healthcare provider groups and larger regional and national health systems.
A critical part of the value proposition of the company’s Cologuard test is its adherence program, which involves active engagement with patients and providers by the company’s adherence team. This customer-oriented support activity is focused on encouraging and helping patients complete Cologuard tests that have been ordered for them by their providers. The company undertakes a variety of activities to promote patient adherence, including letters, text messages, online chat, emails, and phone calls.
The company has undertaken a significant public relations effort to engage patients in the U.S., and launched demographically-targeted, direct-to-patient advertising campaigns in digital, social, print, and other channels. The company promotes its Cologuard test through a national television advertising campaign, with a majority of placements in national cable and syndicated programming widely viewed by the company’s target patient demographic. During 2023, the company continued to deepen its investment in virtual resources, strengthened the company’s digital and television media channels, and explored campaigns to reach 45-49 year old patients.
Precision Oncology Commercial Operations
The company promotes its precision oncology tests through its Precision Oncology sales force. The company’s commercial infrastructure, including its sales force, managed care group, and patient support network, is critical to the success of the company’s precision oncology products. In the company’s domestic sales, marketing and reimbursement efforts, the company interacts directly with medical, radiation, and surgical oncologists, pathologists, and payers. The company employs a direct sales approach that targets oncologists and cancer surgeons, and utilizes medical education and scientific liaisons who target key opinion leaders. The company also plans to continue conducting clinical studies with the objective of having results published in peer-reviewed journals. The combination of these approaches is the company’s best means to increase patient and healthcare provider awareness of the company’s precision oncology products and services and the number of favorable reimbursement coverage decisions by third-party payers.
International Commercial Operations
The company commercializes or plans to commercialize its Oncotype tests outside the United States through employees in Canada, Japan, and a number of European countries, as well as through exclusive distribution agreements. The company has provided its Oncotype tests in approximately 120 countries. The company does not offer its Cologuard test outside of the U.S. The company is exploring opportunities to make available outside of the U.S. the company’s Cologuard test and other products.
Inclusion of the company’s products in guidelines and quality measures will be critical to the company’s international success. The Oncotype DX breast cancer test is recognized in international guidelines issued by the St. Gallen International Breast Cancer Expert Panel and European Society for Medical Oncology. The company’s Oncotype DX breast cancer test has been recommended to guide certain patients’ chemotherapy treatment decisions by the National Institute for Health and Care Excellence in England, the Gynecologic Oncology Working Group in Germany, and the Japan Breast Cancer Society. The company’s Oncotype DX breast cancer test is reimbursed for certain patients in the public health systems in more than ten countries.
The company is exploring opportunities to establish local laboratories in certain locations outside of the U.S. and established local testing capacity in Germany beginning in late 2021. Certain countries have severe restrictions on reimbursing tests performed abroad or exporting tissue samples or patient health data. These restrictions limit the company’s ability to offer its tests in those countries without local laboratories or a method of test delivery that does not require samples to be transported to the company’s U.S. laboratory.
Reimbursement for the company’s Tests
Reimbursement for the company’s Cologuard Test
The company’s Cologuard test has broad reimbursement coverage from Medicare and commercial payers. Updated USPSTF colorectal cancer screening guidelines became final in May 2021 and after a transition period, mandate coverage of the company’s Cologuard test beginning at age 45 for ACA covered health plans. Medicare Part B covers the company’s Cologuard test once every three years for beneficiaries who are age 45 to 85, asymptomatic and at average risk for developing colorectal cancer.
The following laws and regulations establish coverage requirements relevant to the company’s Cologuard test.
Section 2713 of the Patient Protection and Affordable Care Act (‘ACA’) mandates that certain health insurers cover, without imposing any patient cost-sharing, evidence-based items or services that have in effect a rating of ‘A’ or ‘B’ in the current recommendations of USPSTF (‘ACA Mandate’), which includes follow-up colonoscopy after a positive non-invasive stool-based screening test be covered without cost sharing.
Federal regulations require that Medicare Advantage plans cover ‘A’ or ‘B’ rated preventive services without patient cost-sharing, and CMS has issued a notice affirming that Medicare Advantage plans must include coverage of the company’s Cologuard test every three years without patient cost-sharing including coverage of a follow-up colonoscopy after a positive non-invasive stool-based screening test effective January 1, 2023. Additional Part B cost sharing for procedures performed in addition to follow-on colonoscopy (e.g. polyp removal or pathology) will be phased out by 2030.
The laws of approximately 30 states currently mandate coverage of the company’s Cologuard test by certain health insurance plans.
Most commercial payers have issued positive coverage decisions for the company’s Cologuard test, and the company continues to negotiate contracts with payers to include the company’s Cologuard test as an in-network service.
Reimbursement for the company’s Precision Oncology Tests
The company depends on government insurance plans, managed care organizations, and commercial insurance plans for reimbursement of the company’s precision oncology tests.
Medicare coverage for the company’s precision oncology tests is subject to the discretion of the local Medicare Administrative Contractors (‘MAC’). Palmetto, the MAC that establishes the coverage and coding policies for most of the company’s tests under Medicare, developed the Molecular Diagnostic Services Program (‘MolDx’), to identify and establish Medicare coverage for molecular diagnostic tests that fall within the scope of its Molecular Diagnostic Test local coverage decision (‘LCD’). To obtain coverage under the MolDx program, developers of molecular diagnostic tests must submit a detailed dossier of analytical and clinical data to substantiate that a test meets Medicare’s requirements for coverage. The company has received positive coverage decisions under the MolDx program for the company’s breast, colon, Riskguard, and OncoExTra tests.
International Reimbursement
The majority of the company’s international precision oncology revenues come from reimbursement (directly or indirectly), payments from the company’s distributors, and patient self-pay. The company has obtained coverage or other public financing for the company’s breast cancer test outside of the U.S., including coverage for certain patients in Canada, France, Germany, Ireland, Israel, Italy, Japan, the Netherlands, Saudi Arabia, Spain, Sweden, Switzerland, and the United Kingdom (‘U.K.’).
Reimbursement for Future Products
Successful commercialization of the company’s newly developed products will also depend on the company’s ability to obtain and maintain reimbursement at adequate reimbursement rates from government insurance plans, managed care organizations, commercial insurance plans, and public healthcare systems outside the U.S. for such products.
Clinical Laboratory and Manufacturing Facilities
The company processes its Cologuard test at two state of the art, high throughput clinical laboratories in Madison, Wisconsin that are certified pursuant to federal Clinical Laboratory Improvement Amendments (‘CLIA’) and accredited by College of American Pathologist (‘CAP’). The company’s total lab capacity at both facilities is approximately seven million Cologuard tests per year, with the opportunity to add additional capacity, if needed.
The company manufactures its Cologuard test at two facilities in Madison, Wisconsin. The company is committed to manufacturing and providing medical devices and related products that meet customer expectations and applicable regulatory requirements. The company adheres to manufacturing and safety standards required by federal, state, and local laws and regulations; and operates the company’s manufacturing facilities under a quality management system. The company purchases certain components for its Cologuard test from third-party suppliers and manufacturers.
A majority of the company’s internally developed Oncotype tests for domestic and international patients are processed in the company’s CLIA-certified and CAP-accredited clinical reference laboratory facilities in Redwood City, California. Beginning in 2022, Oncotype DX breast cancer tests for German patients have been processed in the company’s newly constructed facility in Trier, Germany, a portion of which is operated by a third-party partner. The company’s OncoExTra tests, along with tests completed under certain of the company’s reference lab agreements, are processed in the company’s CLIA-certified and CAP-accredited clinical reference laboratory facilities in Phoenix, Arizona. The company’s liquid therapy selection tests, which were acquired as part of the company’s acquisition of Resolution Bioscience, are processed in the company’s CLIA-certified and CAP-accredited clinical reference laboratory facility in Kirkland, Washington.
As part of the acquisition of PreventionGenetics, LLC (‘PreventionGenetics’) in December 2021, the company acquired a CLIA-certified and CAP-accredited DNA testing laboratory in Marshfield, Wisconsin. PreventionGenetics’ laboratory provides more than 5,000 predefined genetic tests for nearly all clinically relevant genes, additional custom panels, and comprehensive germline whole exome and whole genome sequencing tests in addition to the company’s hereditary cancer test, Riskguard.
Competition
The company’s precision oncology products compete against a number of companies that offer products or have conducted research to profile genes and gene expression in breast and colon cancer, such as Agendia Inc., Veracyte, Inc., Myriad Genetics, Inc., and Hologic, Inc.
The company faces competition from a variety of sources, including Ambry Genetics, a subsidiary of Konica Minolta Inc.; Myriad Genetics, Inc.; Invitae; Natera; Color Health, Inc.; and Sema4 Genomics, as well as a few large, established general testing companies, such as Laboratory Corporation of America Holdings and Quest Diagnostics Incorporated.
Regulation
Certain of the company’s activities are subject to regulatory oversight by the FDA under provisions of the Federal Food, Drug, and Cosmetic Act (‘FDCA’) and regulations thereunder, including regulations governing the development, marketing, labeling, promotion, manufacturing, distribution, and export of diagnostic products. The company’s clinical laboratory facilities are subject to oversight by CMS pursuant to CLIA, as well as agencies in various states, including New York.
The company’s Cologuard test is regulated by the FDA as a Class III medical device. The FDA granted premarket approval (‘PMA’) for the company’s Cologuard test in August 2014.
Further, the company is required to comply with international, national, and provincial personal data protection laws and regulations, including the European Union's (‘E.U.’) General Data Protection Regulation (‘GDPR’) and Japan's Act on the Protection of Personal Information (‘APPI’).
The company is subject to numerous federal and state anti-fraud and abuse laws, including the Federal False Claims Act.
As the company’s Oncotype DX Breast Recurrence Score test has a pre-existing certification from the company’s notified body, the company had until May 2026 to meet certain of the new, more stringent regulatory requirements of EU IVDR with respect to its Oncotype DX Breast Recurrence Score test, including obtaining a new positive conformity assessment from the company’s notified body. The company received a CE marking on the company’s Oncotype DX Breast Recurrence Score test in December 2023, certifying that the company is in compliance with the new EU IVDR regulatory requirements.
The FCPA prohibits any U.S. individual, business entity, or employee of a U.S. business entity from offering or providing, directly or through a third party, including the distributors the company rely on in certain markets, anything of value to a foreign government official with corrupt intent to influence an award or continuation of business or to gain an unfair advantage, whether or not such conduct violates local laws.
The company’s commercialization activities subject the company to regulations of the Department of Transportation, the U.S. Postal Service, and the Centers for Disease Control and Prevention that apply to the surface and air transportation, as well as importation, of clinical laboratory specimens.
Intellectual Property
As of December 31, 2023, the company had 208 issued patents in the U.S. and 878 issued patents outside of the U.S., which includes validated patents issued by the European Patent Office in key E.U. countries, covering genes and methods that are components of the Cologuard test, Oncoguard Liver test, Oncotype DX tests, pipeline technologies or research methods, and platform technologies. The company’s issued U.S. patents expire at various times between 2024 and 2043. In addition, the company has pending patent applications in the U.S. and in other countries, including provisional and non-provisional filings. Some of these U.S. patent applications also have corresponding pending or granted applications under the Patent Cooperation Treaty in Canada, Europe, Japan, Australia, and other jurisdictions. The company solely owns, jointly owns, or exclusively licenses these patents and patent applications.
License Agreements
Mayo Foundation for Medical Education and Research
In June 2009, the company entered into an exclusive, worldwide license agreement with Mayo Foundation for Medical Education and Research, under which Mayo granted the company an exclusive, worldwide license to certain Mayo patents and patent applications, as well as a non-exclusive, worldwide license with regard to certain Mayo know-how. The scope of the license covers any screening, surveillance, or diagnostic test or tool for use in connection with any type of cancer, pre-cancer, disease, or condition. The company’s license agreement with Mayo was most recently amended and restated in September 2020.
The licensed Mayo patents and patent applications contain both method and composition claims that relate to sample processing, analytical testing, and data analysis associated with nucleic acid screening for cancers and other diseases. The jurisdictions covered by these patents and patent applications include the U.S., Australia, Canada, the E.U., China, Japan, and Korea. Under the license agreement, the company assumed the obligation and expense of prosecuting and maintaining the licensed Mayo patents and are obligated to make commercially reasonable efforts to bring to market products using the licensed Mayo intellectual property.
Pursuant to the company’s license agreement with Mayo, the company is required to pay Mayo a low-single-digit royalty on net sales of products using the licensed Mayo intellectual property each year during the term of the Mayo agreement.
The license agreement will remain in effect, unless earlier terminated by the parties in accordance with the agreement, until the last of the licensed patents expires in 2043 (or later, if certain licensed patent applications are issued).
In addition to granting the company a license to the covered Mayo intellectual property, Mayo provides the company with product development research and development assistance pursuant to the license agreement and other collaborative arrangements. In September 2020, Mayo also agreed to make available certain personnel to provide, such assistance through January 2025.
Johns Hopkins University
Through the acquisition of Thrive Earlier Detection Corporation (‘Thrive’), the company acquired a worldwide exclusive license agreement with JHU for use of several JHU patents and licensed know-how. The company is seeking to utilize the JHU licensed technology to develop and commercialize a blood-based MCED test.
In addition to granting the company a license to the covered JHU intellectual property, JHU provides the company with research and development assistance pursuant to other collaboration arrangements.
History
Exact Sciences Corporation was founded in 1995. The company was incorporated in the state of Delaware in 1995.

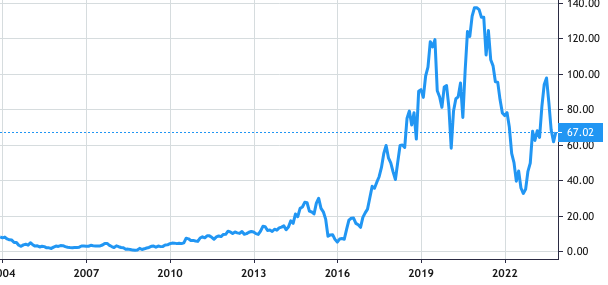
 Stock Value
Stock Value

