About Zoetis
Zoetis Inc. (Zoetis) operates as a global leader in the animal health industry.
The company focuses on the discovery, development, manufacture and commercialization of medicines, vaccines, diagnostic products and services, biodevices, genetic tests and precision animal health.
The company has a diversified business, commercializing products across eight core species: dogs, cats and horses (collectively, companion animals) and cattle, swine, poultry, fish and sheep (collectively, livestock); and within seven major product categories: parasiticides, vaccines, dermatology, other pharmaceutical, anti-infectives, animal health diagnostics and medicated feed additives.
Operating Segments
The company organizes and operates its business in two segments: United States (U.S.) and International. Within each of these operating segments, the company offers a diversified product portfolio for both companion animal and livestock customers so that the company can capitalize on local trends and customer needs.
In addition, the company’s Client Supply Services (CSS) organization, which provides contract manufacturing services to third parties, and the company’s human health products, together represented approximately 1% of the company’s total revenue for the year ended December 31, 2023.

Products
The company focuses on developing a diverse portfolio of animal health products that deliver solutions across the continuum of care. The company refers to all different brands of a particular product, or its dosage forms for all species, as a product line. The company has approximately 300 comprehensive product lines, including products for both companion animals and livestock within each of the company’s major product categories.
The company’s companion animal products help extend and improve the quality of life for pets; increase convenience and compliance for pet owners; and help veterinarians improve the quality of their care and the efficiency of their businesses. Companion animals are also living longer, deepening the human-animal bond, receiving increased medical treatment and benefiting from advances in animal health medicines, vaccines and diagnostics. Companion animal products represented approximately 65% of the company’s revenue for the year ended December 31, 2023.
The company’s livestock products focus on predicting, preventing, detecting and treating diseases, with the understanding that veterinarians and farmers must have the tools at their disposal to treat disease expeditiously, which in turn enables the sustainable production of safe, high-quality animal protein. Livestock products represented approximately 34% of the company’s revenue for the year ended December 31, 2023.
In addition, the company’s CSS organization, which provides contract manufacturing services to third parties, and the company’s human health products, together represented approximately 1% of the company’s total revenue for the year ended December 31, 2023.
The company’s major product categories are:
Parasiticides: Products that prevent or eliminate external and internal parasites, such as fleas, ticks and worms;
Vaccines: Biological preparations that help prevent diseases of the respiratory, gastrointestinal and reproductive tracts or induce a specific immune response;
Dermatology: Products that relieve itch associated with allergic conditions and atopic dermatitis;
Other Pharmaceutical: Pain and sedation, antiemetic, reproductive, and oncology products;
Anti-Infectives: Products that kill or slow the growth of bacteria, fungi or protozoa;
Animal Health Diagnostics: Testing and analysis of blood, urine and other animal samples and related products and services, including point-of-care diagnostic products, instruments and reagents, rapid immunoassay tests, reference laboratory kits and services and blood glucose monitors; and
Medicated Feed Additives: Products added to animal feed that provide medicines to livestock.
The company’s remaining revenue is derived from other non-pharmaceutical product categories, such as nutritionals, as well as products and services in biodevices, genetic tests and precision animal health.
As part of the company’s growth strategy, the company focuses on the discovery and development of new chemical, biopharmaceutical and biological entities, as well as product lifecycle innovation, primarily through the company’s research and development (R&D) group. Historically, a substantial portion of the company’s products and revenue has been the result of product lifecycle innovation where the company actively works to broaden the value of existing products by developing claims in additional species, more convenient formulations and combinations, and by expanding usage into more countries. For example, the first product in the company’s ceftiofur line was an anti-infective approved for treating bovine respiratory disease (BRD) in cattle that was administered via intramuscular injection. Through follow-on studies and reformulations, the company has expanded the product line into additional cattle claims and administration routes, as well as other species and regions. The ceftiofur product line includes the brands Excede, Excenel, Naxcel and Spectramast.
The following are examples of the company’s first-in-class and/or best-in-class products that the company has launched in recent years and products that represent platforms for future product lifecycle innovation:
Apoquel, the first Janus kinase inhibitor for use in veterinary medicine, was approved in 2013 for the control of pruritus associated with allergic dermatitis and the control of atopic dermatitis in dogs at least 12 months of age. Since January 2014, the company has launched Apoquel in many key markets globally. In 2021, a chewable version of Apoquel was approved in the European Union (EU) and the United Kingdom (U.K.), and has since been approved in other key markets globally, including the U.S. in 2023;
Core EQ Innovator, the first and only vaccine for horses to contain all five core equine disease antigens - West Nile, Eastern and Western Equine encephalomyelitis, tetanus and rabies - in one combination, was approved in the U.S. in 2018 and in Canada in 2019;
Cytopoint, the first canine monoclonal antibody (mAb) to help reduce the clinical signs of atopic dermatitis (such as itching) in dogs of any age, was licensed in the U.S. in 2016 (and was later granted an expanded indication to treat allergic dermatitis in 2018). The product has since been approved in many key markets globally. An injection given once every four to eight weeks, Cytopoint neutralizes interleukin-31, a protein that has been demonstrated to trigger itching in dogs;
Fostera PCV MH was introduced in November 2013 in the U.S. and has since been approved in many key markets globally. It was developed to help protect pigs from porcine circovirus-associated disease (PCVAD) and enzootic pneumonia caused by M. hyopneumoniae (M. hyo). Fostera Gold PCV MH, the only vaccine to contain two PCV2 genotypes and long-lasting M. hyo coverage, was approved in the U.S. and Canada in 2018 and has since been approved in many key markets globally. The Fostera franchise also includes Fostera/Suvaxyn PRRS, which was approved in the U.S. in 2015 and has since been approved in many key markets globally. This vaccine offers protection against both the respiratory and reproductive forms of disease caused by porcine reproductive and respiratory syndrome (PRRS) virus;
Librela (bedinvetmab), the first and only injectable mAb therapy for monthly alleviation of osteoarthritis (OA) pain in dogs, was approved in the EU in 2020 and has since been approved in other key markets globally, including the U.S. in 2023;
Poulvac Procerta HVT-ND, the company’s first vector vaccine that helps protect against Marek’s disease and Newcastle disease, highly contagious viral infections affecting poultry, was approved in the U.S. in 2019. In 2020, the company expanded its line of recombinant vector vaccines with the launch of Poulvac Procerta HVT-IBD, which helps protect against Marek's disease and provides early protection against the contemporary infectious bursal disease (IBD) viruses. The company has since expanded Poulvac Procerta HVT-ND into other key markets globally. In 2022, the company further expanded its line of recombinant vector vaccines with the launch of Poulvac Procerta HVT-IBD-ND in the U.S., which is an advanced trivalent vector vaccine that delivers powerful early protection against Marek's disease, infectious bursal disease and Newcastle disease in one dose;
ProHeart 12 (moxidectin), a once-yearly injection to prevent heartworm disease in dogs 12 months of age and older, was approved in the U.S. in 2019;
Protivity, a modified-live bacterial vaccine that is effective in protecting healthy beef and dairy cattle against respiratory disease caused by Mycoplasma bovis (M. bovis), was approved in the U.S. and Canada in 2022 and in the EU and Mexico in 2023;
Simparica (sarolaner) Chewables, a monthly chewable tablet for dogs to control fleas and ticks, was approved in the EU in 2015 and has since been approved in other key markets globally, including the U.S. Simparica Trio, a triple combination parasiticide for dogs, was approved in the EU and Canada in 2019 and has since been approved in other key markets globally, including the U.S., and received approval in 2023 in other key markets globally for new claims related to the prevention of eyeworms, Otodectes cynotis, flea tapeworms and efficacy against sarcoptic and demodectic manges. This product is a key internal lifecycle innovation that combines flea and tick treatment (sarolaner) with the prevention of heartworm disease and treatment of gastrointestinal parasites;
Solensia (frunevetmab), the first injectable mAb therapy for monthly alleviation of OA pain in cats, was approved in Switzerland in 2020 and has since been approved in other key markets globally, including the EU and the U.S.;
Stronghold Plus (selamectin/sarolaner), also referred to as Revolution Plus in certain jurisdictions, a topical combination product that treats ticks, fleas, ear mites, lice and gastrointestinal worms and prevents heartworm disease in cats, received EU approval in 2017 and has since been approved in other key markets globally, including the U.S.; and
Vanguard/Versican is a market leading vaccine line for dogs intended to help prevent a range of diseases. Since 2016, Zoetis has added new and innovative enhancements to its Vanguard line in the U.S. with Vanguard crLyme, Vanguard Rapid Resp Intranasal, Vanguard B Oral, Vanguard CIV H3N2/H3N8 and Versican Plus Bb Oral.
The company pursues the development of new vaccines for emerging infectious diseases, with an operating philosophy of ‘first to know and fast to market’. Examples of the successful execution of this strategy include the first SARS-CoV-2 (COVID-19) vaccine to help protect the health and well-being of more than 300 mammalian species living in zoos, aquariums, conservatories and other animal organizations around the world; the first equine vaccine for West Nile virus in the U.S. and EU; the first swine vaccine for pandemic H1N1 influenza virus in the U.S.; the first licensed vaccine against the pandemic H5N1 bird flu in the U.S. and EU, which the company provided to the U.S. Department of Agriculture when it recommended the company’s vaccine be used by the U.S. Fish and Wildlife Service to help protect California condors; a conditionally licensed vaccine to help fight porcine epidemic diarrhea virus (PEDv) in the U.S.; and the first conditionally licensed vaccine to help prevent the H3N2 type of canine influenza that emerged in the U.S. In 2019, Zoetis established a research facility with Texas A&M University to develop vaccines for transboundary and emerging diseases in animals, including Foot-and-Mouth Disease (FMD), a virus that can cause serious illness in cattle, pigs, and sheep. In 2020, the company opened a research lab at Colorado State University in a partnership to increase the company’s understanding of the potential use of immunomodulators in livestock that could reduce the need for antibiotics, as well as advance the company’s understanding of the biology of key diseases affecting companion animals which could lead to new therapies that can treat chronic health conditions in pets.
Additionally, the Pharmaq business of Zoetis is the global leader in vaccines and innovation for aquatic health products, with its leading Alpha Ject vaccine line, Alpha Flux, a parasiticide that helps salmon farmers in Chile control sea lice infestations, one of the major challenges in the aquatic health industry and Alpha ERM Salar, a water-based injectable vaccine that helps protect salmon from red mouth, a common bacterial infection.
Zoetis enhanced the portfolio of its diagnostic products with the acquisition in 2018 of Abaxis, Inc. (Abaxis), a leading provider of veterinary point-of-care diagnostic instruments. With this acquisition came the VetScan portfolio of benchtop and handheld diagnostic instruments and consumables, which serves a large customer base of veterinary practices both in the U.S. and international markets. In 2019, the company acquired Phoenix Central Laboratory for Veterinarians, Inc. and ZNLabs, LLC marking its entry into reference laboratory services and building on a strategy to develop a more comprehensive diagnostics offering with enhanced value for veterinarians. In 2020, the company acquired a third veterinary reference lab business, Ethos Diagnostic Science. The Zoetis diagnostic portfolio also includes the Witness, Serelisa and ProFlok lines of immunodiagnostic kits, which provide disease detection capabilities for various species, including dogs, cats, cattle, swine and poultry. In 2020, the company launched Vetscan Imagyst, which uses a combination of image recognition technology, algorithms and cloud-based artificial intelligence (AI) to deliver rapid testing results to veterinary clinics. In 2021, the company added digital cytology testing to the Vetscan Imagyst platform. In 2022, the company added AI blood smear testing to the Vetscan Imagyst platform. In 2023, the company added AI dermatology and AI fecal for equine to the Vetscan Imagyst platform. As Zoetis continues to develop additional innovative applications for Vetscan Imagyst, it plans to seamlessly integrate even more new capabilities into the platform, helping veterinarians provide the best possible care for animals. Also in 2023, the company added Vetscan Mastigram+ for rapid, on-farm mastitis diagnosis for dairy cows.
Zoetis also entered the field of animal nutritionals with the acquisition of Platinum Performance in 2019. The acquisition brings the company premium nutritional product formulas and a unique approach to the field of scientific wellness for horses, dogs and cats.
In 2022, the company completed the acquisition of Jurox, an animal health company based in Australia, which brings the company a range of companion animal and livestock products and provides the company with future growth opportunities, manufacturing capacity and increased capabilities in Australia. Also in 2022, the company acquired Basepaws, a petcare genetics company that provides pet owners with genetic tests, analytics and early health risk assessments, which help pet owners and veterinarians understand an individual pet’s risk for disease and helps Zoetis identify solutions to complex diseases by informing the company’s research and innovation.
In 2023, the company completed the acquisition of PetMedix Ltd, a privately held research and development stage animal health biopharmaceutical company based in the U.K., which develops antibody-based therapeutics for companion animals. Also in 2023, the company completed the acquisition of adivo GmbH, a privately held research and development stage animal health biopharmaceutical company based in Germany.
In 2023, the company’s two top-selling products and product lines, Simparica/Simparica Trio and Apoquel/Apoquel Chewable, contributed approximately 13% and 10%, respectively, of the company’s revenue. Combined with the company’s next three top-selling products and product lines, Cytopoint, Revolution/Revolution Plus/Stronghold and ceftiofur line, these five products and product lines contributed approximately 37% of the company’s revenue. In 2023, the company’s ten top-selling products and product lines contributed approximately 49% of the company’s revenue. In 2023, the company had 15 products and product lines.
International Operations
The company directly markets its products in approximately 45 countries across North America, Europe, Africa, Asia, Australia, and South America. The company’s products are sold in more than 100 countries. Operations outside the U.S. accounted for 46% of the company’s total revenue for the year ended December 31, 2023. Through the company’s efforts to establish an early and direct presence in many emerging markets, such as Brazil, Chile, China and Mexico, emerging markets contributed 21% of the company’s total revenue for the year ended December 31, 2023.
Sales and Marketing
The company’s sales organization includes sales representatives and technical and veterinary operations specialists. The company also contracts with distributors that provide logistics and sales and marketing support for many of the company’s products. In regions where the company does not maintain a direct commercial presence, the company relies solely on distributors for these services.
The company’s sales representatives visit its customers, including veterinarians and livestock producers, to provide information and to promote and sell the company’s products and services. The company’s technical and veterinary operations specialists, who generally have advanced veterinary medicine degrees, provide scientific consulting focused on disease management and herd management, training and education on diverse topics, including responsible product use. These direct relationships with customers allow the company to understand the needs of its customers. Additionally, the company’s sales representatives and technical and veterinary operations specialists partner with customers to provide training and support in areas of disease awareness and treatment protocols, including the use of the company’s products. As a result of these relationships, the company’s sales and consulting visits are typically longer, more meaningful and provide the company with better access to customer decision makers as compared to those in human health. In certain markets, including the U.S., pet owners are taking a more active role in product purchasing decisions, and as a result the company is increasingly investing in direct-to-consumer marketing efforts. As of December 31, 2023, the company’s sales organization consisted of approximately 4,100 employees.
The company’s companion animal and livestock products are primarily available by prescription through a veterinarian. On a more limited basis, in certain markets, the company sells certain products through retail and e-commerce outlets. The company also markets its products by advertising to veterinarians, livestock producers, and pet owners.
Customers
The company primarily sells its companion animal products to veterinarians or to third-party veterinary distributors that typically then sell the company’s products to veterinarians, and in each case veterinarians then typically sell the company’s products to pet owners. In certain markets, the company also sells certain companion animal products through retail and e-commerce outlets. The company sells its livestock products primarily to veterinarians and livestock producers, including beef and dairy farmers, as well as pork and poultry operators, in addition to third-party veterinary distributors and retail outlets who then typically sell the products to livestock producers. Sales to the company’s largest customer, a U.S. veterinary distributor, represented approximately 15% of total revenue for 2023.
Research and Development (R&D)
The company incurred R&D expenses of $614 million in 2023.
Intellectual Property
The company’s product portfolio enjoys the protection of approximately 5,880 granted patents and 1,490 pending patent applications, filed in more than 50 countries, with a focus on the company’s major markets, including Australia, Brazil, Canada, China, Europe, Japan and the U.S., as well as other countries with strong patent systems.
Below is a summary of the company’s recent and upcoming key patent expirations.
Patents relating to the active ingredient (tulathromycin) and formulation of Draxxin have expired in the majority of markets. Exceptions are the formulation patent that expires in November 2025 in Japan, and formulation and active ingredient patents in Brazil which expire in July and December 2025, respectively. Generic or other competing tulathromycin products are now marketed in most major markets, as well as in many smaller markets. Additional marketing authorizations for generic tulathromycin products may be granted in various markets in the future. Sales of Draxxin have been negatively affected by generic competition in the markets where the patents have expired.
All patents relating to the active ingredient of Excede/Naxcel (ceftiofur crystalline free acid) have expired. The patents covering the commercial formulation of Excede in the U.S., Japan and Brazil extend to July 2024, September 2026 and August 2027, respectively, but the corresponding patents in Europe, Canada and Australia expired in September 2021. The commercial method of administration patent relevant to the product line expired in March 2023 in the U.S., Europe and Australia, and will expire in March 2028 in Japan. Generic versions of Excede have entered the market in Brazil, Mexico and Russia. At this time, the market entry of a generic version of Excede in the U.S. is not anticipated before July 2024.
All patents relating to Revolution/Stronghold containing selamectin as the sole active ingredient have expired. Generic versions of selamectin are now sold in markets, including the U.S., Europe, Australia and Canada. Selamectin is one of the active ingredients in the company’s combination parasiticide product, Revolution Plus/Stronghold Plus, which is separately patent protected.
The patent for the active ingredient of Convenia (cefovecin sodium) has expired in all countries, the patents covering the commercial formulation expired in all countries in November 2022, except for the U.S., which expired in October 2023 and Brazil, which will expire in November 2025.
The patent for the active ingredient of Cerenia has expired in all countries and the formulation patents relevant to the injectable product line expire between 2025 and 2028. Generic versions of Cerenia injectable have been registered and marketed in Europe, Canada and Australia; and the company is aware that regulatory approval of at least one generic version of Cerenia injectable is being pursued in the U.S.
The company maintains more than 10,500 trademark applications and registrations in its market countries, identifying products and services dedicated to the care of companion animals and livestock.
Regulatory
The United States
As a result of the company’s acquisition of Abaxis, the company’s product portfolio includes human medical devices, which are subject to regulation in the U.S. by the FDA under the Federal Food, Drug, and Cosmetic Act, including the 1976 Medical Device Amendments and the Quality System Regulation, and the Clinical Laboratory Improvement Amendments of 1988, and by the Department of Health and Human Services Office for Civil Rights under the Health Insurance Portability and Accountability Act of 1996. Post-market surveillance is required, with reports provided to the FDA in accordance with agency requirements.
Zoetis is also subject to foreign trade controls administered by certain U.S. government agencies, including the Bureau of Industry and Security within the Commerce Department, Customs and Border Protection within the Department of Homeland Security and the U.S. Department of the Treasury’s Office of Foreign Assets Control (OFAC). As a global animal health company, the company conducts business in multiple jurisdictions throughout the world. This includes supplying medicines and medical products for use in Iran and shipment of products to Iran, and conducting related activities, in accordance with a general license issued by OFAC and in line with the company’s corporate policies. As previously disclosed, the company acquired Platinum Performance (Platinum) in August 2019. During the integration process, after the closing of the acquisition, the company discovered that Platinum had initiated certain transactions involving sales of food, medicine or devices to individuals or entities who may have been resident in or had ties to Iran. These sales were not conducted pursuant to a general license from OFAC and potentially violated the Iranian Transactions and Sanctions Regulations (ITSR) administered by OFAC. The company submitted an initial voluntary disclosure to OFAC in February 2020 while the company’s internal investigation was ongoing. After concluding the company’s internal investigation, in December 2020, the company submitted a final voluntary disclosure to OFAC and the U.S. Department of Justice regarding these transactions. In July 2023, OFAC provided a No Action letter confirming a final determination that no further action would be taken in the matter. To date, the Department of Justice has not responded. In addition, the company is subject to a wide variety of state level regulations in the United States covering topics, such as the environment, animal welfare and privacy.
Outside the United States
The company is also subject to the EU General Data Protection Regulation (GDPR) that requires the company to meet enhanced requirements regarding the handling of personal data, including its use, protection and the rights of data subjects to request correction or deletion of their personal data.
Global Policy and Guidance
The Joint FAO/WHO Expert Committee on Food Additives is an international expert scientific committee that is administered jointly by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO). They provide a risk assessment/safety evaluation of residues of veterinary drugs in animal products, exposure and residue definition and maximum residue limit proposals for veterinary drugs. The company works with them to establish acceptable safe levels of residual product in food-producing animals after treatment. This in turn enables the calculation of appropriate withdrawal times for the company’s products prior to an animal entering the food chain.
Competition
The company’s primary competitors include animal health medicines, vaccines and diagnostic companies, such as Boehringer Ingelheim Animal Health Inc., the animal health division of Boehringer Ingelheim GmbH; Merck Animal Health, the animal health division of Merck & Co., Inc.; Elanco Animal Health; and IDEXX Laboratories.
History
Zoetis Inc. was founded in 1952. The company was incorporated in Delaware in 2012.

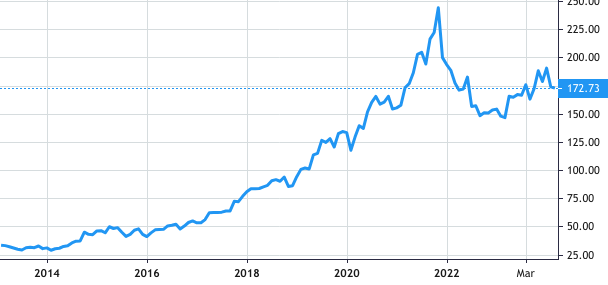
 Stock Value
Stock Value

