About Pfizer
Pfizer Inc. (Pfizer) operates as a research-based, global biopharmaceutical company.
The company applies science and its global resources to bring therapies to people that extend and significantly improve their lives through the discovery, development, manufacture, marketing, sale, and distribution of biopharmaceutical products worldwide.
The company works across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of its time. The company collaborates with healthcare providers, governments and local communities to support and expands access to reliable, affordable healthcare around the world.
Most of the company's revenues come from the manufacture and sale of biopharmaceutical products. The company also sells products for the detection of certain illnesses and provide end-to-end R&D services to select innovative biotech companies. The company's medicines and vaccines provide significant value for healthcare providers and patients through improved treatment of diseases and improvements in health, wellness and productivity as well as by reducing other healthcare costs, such as emergency room visits or hospitalizations. The company seeks to enhance the value of its medicines and vaccines and actively engage in dialogues about how it can best work with patients, physicians and payors to prevent and treat disease and improve outcomes.
In addition, Pfizer's ESG strategy, which is integrated into its corporate strategy, focuses on six areas where the company sees opportunities to create a meaningful impact: product innovation; equitable access and pricing; product quality and safety; diversity, equity and inclusion; climate change; and business ethics.
The company is committed to strategically capitalizing on growth opportunities, primarily by advancing its own product pipeline and maximizing the value of its existing products, but also through various business development activities. The company views its business development activity as an enabler of its strategies and seek to generate growth by pursuing opportunities and transactions that have the potential to strengthen its business and its capabilities. The company assesses its business, assets, and scientific capabilities/portfolio as part of its regular, ongoing portfolio review process and also continue to consider business development activities that will help advance its business strategy.

On December 14, 2023, the company completed its acquisition of Seagen (Seagen Inc. and its subsidiaries), a global biotechnology company that discovers, develops and commercializes transformative cancer medicines. With the addition of Seagen's pipeline and its four in-line medicines (Padcev, Adcetris, Tukysa and Tivdak), Pfizer's oncology portfolio spans multiple modalities, including ADCs, small molecules, bispecifics and other immunotherapies. In addition to the acquisition of Seagen, the company's significant recent business development activities in 2023 include, among others, the September 2023 divestiture of its early-stage rare disease gene therapy portfolio to Alexion.
Commercial Operations
In 2023, the company managed its commercial operations through a global structure consisting of two operating segments, each led by a single manager: Biopharma, its innovative science-based biopharmaceutical business, and Business Innovation, an operating segment established in the first quarter of 2023 that includes PC1, its contract development and manufacturing organization and a leading supplier of specialty active pharmaceutical ingredients, and Pfizer Ignite, an offering that provides strategic guidance and end-to-end R&D services to select innovative biotech companies that align with its R&D focus areas. In 2023, Biopharma was the only reportable segment. The commercial structure within Biopharma included three broad customer groups in 2023: Primary Care, Specialty Care and Oncology.
At the beginning of 2024, the company made changes in its commercial organization to incorporate Seagen and improve focus, speed and execution. Specifically, within its Biopharma reportable segment it created the Pfizer Oncology Division, the Pfizer U.S. Commercial Division, and the Pfizer International Commercial Division.
Pfizer Oncology Division
Combines the U.S. Oncology commercial organizations, global Oncology marketing organizations and global and U.S. Oncology medical affairs from both Pfizer and Seagen. Includes innovative oncology product portfolio of ADCs, small molecules, bispecifics and other immunotherapies that treat a wide range of cancers, including certain types of breast cancer, genitourinary cancer and hematologic malignancies, as well as certain types of melanoma, gastrointestinal, gynecological and thoracic cancers, which includes lung cancer.
Pfizer U.S. Commercial Division
Includes the U.S. Primary Care and U.S. Specialty Care customer groups, the Chief Marketing Office, the Global Chief Medical Affairs Office and Global Access & Value.
U.S. Primary Care includes internal medicine product portfolio of brands in cardiovascular metabolic, bone graft for spinal fusion and women's health, as well as post-LOE brands; migraine product portfolio; vaccines product portfolio across all ages with a pipeline focus on infectious diseases with significant unmet medical need, including COVID-19 (novel coronavirus disease of 2019); treatment for COVID-19; and products for detection of COVID-19 and influenza.
U.S. Specialty Care includes inflammation & immunology product portfolio of brands and biosimilars for chronic immune and inflammatory diseases; rare disease product portfolio of brands for a number of therapeutic areas with rare diseases, including amyloidosis, hemophilia, endocrine diseases and sickle cell disease; and hospital product portfolio of sterile injectable and immunoglobulin medicines.
Pfizer International Commercial Division
Includes the ex-U.S. commercial and medical affairs organizations covering Pfizer's entire product portfolio in all international markets.
Primary Care:
Internal medicine: Eliquis, the Premarin family and BMP2.
Migraine: Nurtec ODT/Vydura and Zavzpret.
Vaccines: Comirnaty, the Prevnar family, Abrysvo, FSME/IMMUN-TicoVac, Nimenrix and Trumenba.
Treatment for COVID-19: Paxlovid.
Detection of COVID-19 and influenza: Lucira by Pfizer.
Select products within Oncology, Primary Care and Specialty Care include:
Oncology: Ibrance, Xtandi, Inlyta, Bosulif, Lorbrena, Braftovi, Mektovi, Padcev, Adcetris, Talzenna, Tukysa, Elrexfio and Tivdak.
Specialty Care:
Inflammation & immunology: Xeljanz, Enbrel (outside the U.S. and Canada), Inflectra, Cibinqo, Litfulo and Velsipity.
Rare disease: The Vyndaqel family, Genotropin, BeneFIX, Oxbryta, Somavert and Ngenla.
Hospital: Sulperazon, Zavicefta, Zithromax, Medrol and Panzyga.
Collaboration and Co-Promotion Agreements
The company uses collaboration and/or co-promotion arrangements to enhance its development, R&D, sales and distribution of certain biopharmaceutical products, which include, among others, the following:
Comirnaty is an messenger ribonucleic acid (mRNA) based coronavirus vaccine to help prevent COVID-19, which is being jointly developed and commercialized with BioNTech SE (BioNTech). Pfizer and BioNTech equally share the costs of development for the Comirnaty program. Comirnaty has been granted an approval or an authorization in many countries around the world in populations varying by country.
Eliquis (apixaban) is part of the Novel Oral Anticoagulant market and was jointly developed and commercialized with BMS as an alternative treatment option to warfarin in appropriate patients.
Xtandi (enzalutamide) is an androgen receptor inhibitor that blocks multiple steps in the androgen receptor signaling pathway within tumor cells that is being developed and commercialized in collaboration with Astellas.
Orgovyx (relugolix) is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist for the treatment of adult patients with advanced prostate cancer that is being developed and commercialized with SMPA. The companies are also collaborating on Myfembree (relugolix 40 mg, estradiol 1.0 mg, and norethindrone acetate 0.5 mg) for heavy menstrual bleeding associated with uterine fibroids in premenopausal women and the management of moderate to severe pain associated with endometriosis in premenopausal women. The companies equally share profits and allowable expenses in the U.S. for Orgovyx, and in the U.S. and Canada for Myfembree.
Padcev (enfortumab vedotin-ejfv) is a first-in-class ADC that is directed to Nectin-4, a protein located on the surface of cells and highly expressed in bladder cancer, that is being co-developed and jointly commercialized with Astellas. In the U.S., Padcev has been approved for use with Keytruda (pembrolizumab) for adult patients with locally advanced or metastatic urothelial cancer. Other approvals and indications for Padcev vary by market. In the U.S., the companies jointly promote, and the company record net sales and are responsible for all U.S. distribution activities for Padcev. Outside the U.S., the company has commercialization rights in all countries in North and South America, and Astellas has commercialization rights in the rest of the world.
In addition, the company has collaboration and/or co-promotion arrangements with respect to certain other biopharmaceutical products, including Adcetris and Tivdak as a result of its acquisition of Seagen.
Revenues associated with these arrangements are included in Alliance revenues (except in certain markets where the company has direct sales and except for the majority of revenues for Comirnaty and Padcev, which are included in Product revenues). In addition, the company has collaboration arrangements for the development and commercialization of certain pipeline products that are in development stage.
International Operations
The company's operations are conducted globally, and it supplies its medicines and vaccines to approximately 200 countries and territories
Sales and Marketing
The company's prescription biopharmaceutical products, with the exception of Paxlovid in 2022 and 2023, are sold principally to wholesalers, but it also sells directly to retailers, hospitals, clinics, government agencies and pharmacies. In 2023, the company principally sold Paxlovid globally to government agencies. The company's vaccines in the U.S. are primarily sold directly to the federal government (including the CDC), wholesalers, individual provider offices, retail pharmacies and integrated delivery systems. The company's vaccines outside the U.S. are primarily sold to government and non-government institutions. Certain of these government contracts may be renegotiated or terminated at the discretion of a government entity. The company's contracts with government and supranational organizations for the sales of Comirnaty and Paxlovid, which are binding contracts, represented a significant amount of revenues in 2023. Sales of Comirnaty and Paxlovid in the U.S. transitioned to commercial channels in the second half of 2023.
The company promotes its products to healthcare providers and patients consistent with applicable laws. Through its marketing organizations, the company explains the approved uses, benefits and risks of its products to healthcare providers and patients; MCOs that provide insurance coverage, such as hospitals, integrated delivery systems, PBMs and health plans; and employers and government agencies who hire MCOs to provide health benefits to their employees. In the U.S., the company markets directly to consumers through direct-to-consumer advertising that seeks to communicate the approved uses, benefits and risks of its products while motivating people to have meaningful conversations with their doctors. In addition, the company sponsors general advertising to educate the public on disease awareness, prevention and wellness, important public health issues and its patient assistance programs.
As part of its commitment to engaging its customers in a manner they prefer, the company takes an omnichannel approach, including both virtual and in person interactions, and sees generally positive customer response to both approaches.
Patents and Other Intellectual Property Rights
Patents: The company owns or has co-promotion and/or license rights related to a number of patents covering pharmaceutical and other products, their uses, formulations, and product manufacturing processes.
Government Regulation
The U.S. Food and Drug Administration (FDA), pursuant to the U.S. Federal Food, Drug and Cosmetic Act (FFDCA), the Public Health Service Act and other federal statutes and regulations, extensively regulates pre- and post-marketing activities related to the company's biopharmaceutical products. Other the U.S. federal agencies, including the U.S. Drug Enforcement Agency (DEA), also regulate certain of the company's products and activities.
The company is also required to report adverse events and comply with current Good Manufacturing Practices (cGMPs) (the FDA regulations that govern all aspects of manufacturing quality for pharmaceuticals) and the Drug Supply Chain Security Act (the law that among other things, sets forth requirements related to product tracing, product identifiers and verification for manufacturers, wholesale distributors, re-packagers and dispensers to facilitate the tracing of product through the pharmaceutical distribution supply chain), as well as advertising and promotion regulations.
The company's marketing practices are subject to state laws, as well as federal laws, such as the Anti-Kickback Statute and False Claims Act, intended to prevent fraud and abuse in the healthcare industry. In the European Union (EU), the European Medicines Agency (EMA) conducts the scientific evaluation, supervision and safety monitoring of the company's innovative medicinal products that are eligible for the centralized marketing authorization procedure
History
Pfizer Inc. was founded in 1849. The company was incorporated under the laws of the state of Delaware in 1942.

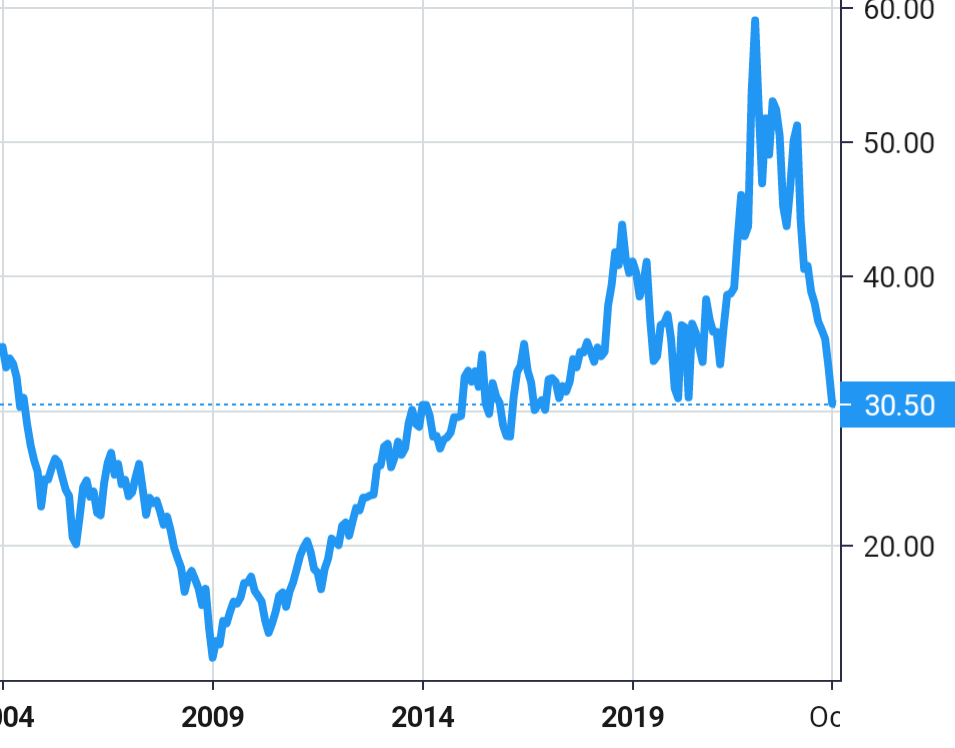
 Stock Value
Stock Value

