About Eli Lilly and
Eli Lilly and Company discovers, develops, manufactures, and markets products in human pharmaceutical products. Most of the products that the company sells are discovered or developed by its own scientists, and its long-term success depends on its ability to continually discover or acquire, develop, and commercialize innovative medicines.
The company manufactures and distributes its products through facilities in the United States (U.S.), including Puerto Rico, and in Europe and Asia. The company’s products are sold in approximately 105 countries.
Products
The company’s products include:
Diabetes, Obesity and Other Cardiometabolic Products
Basaglar: In collaboration with Boehringer Ingelheim, a long-acting human insulin analog for the treatment of diabetes.

Humalog, Humalog Mix 75/25, Humalog U-100, Humalog U-200, Humalog Mix 50/50, insulin lispro, insulin lispro protamine, and insulin lispro mix 75/25: Certain indications include human insulin analogs for the treatment of diabetes.
Humulin, Humulin 70/30, Humulin N, Humulin R, and Humulin U-500: Human insulins of recombinant DNA origin for the treatment of diabetes.
Jardiance: In collaboration with Boehringer Ingelheim, for the treatment of type 2 diabetes; to reduce the risk of cardiovascular death in adult patients with type 2 diabetes and established cardiovascular disease; to reduce the risk of cardiovascular death and hospitalizations for heart failure in adults; and to reduce the risk of sustained decline in estimated glomerular filtration rate (eGFR), end-stage kidney disease, cardiovascular death and hospitalization in adults with chronic kidney disease (CKD) at risk of progression.
Mounjaro: A glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist, for the treatment of adults with type 2 diabetes in combination with diet and exercise to improve glycemic control.
Trulicity: For the treatment of type 2 diabetes in adults and pediatric patients 10 years of age and older; and to reduce the risk of major adverse cardiovascular events in adult patients with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors.
Zepbound: For the treatment of adults with obesity or overweight with weight-related comorbidities as an adjunct to a reduced-calorie diet and increased physical activity (marketed under Mounjaro in the European Union (EU) and in various other markets outside the U.S.).
Oncology Products
Marketing and Distribution
Alimta: For the first-line treatment, in combination with two other agents, of advanced non-small cell lung cancer (NSCLC) for patients with non-squamous cell histology and no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations; for the first-line treatment, in combination with another agent, of advanced non-squamous NSCLC; for the second-line treatment of advanced non-squamous NSCLC; as monotherapy for the maintenance treatment of advanced non-squamous NSCLC in patients whose disease has not progressed immediately following chemotherapy treatment; and in combination with another agent for the treatment of malignant pleural mesothelioma.
Cyramza: For use as monotherapy or in combination with another agent as a second-line treatment of advanced or metastatic gastric cancer or gastro-esophageal junction adenocarcinoma; in combination with another agent as a second-line treatment of metastatic NSCLC; in combination with another agent as a second-line treatment of metastatic colorectal cancer; as a monotherapy as a second-line treatment of hepatocellular carcinoma; and in combination with another agent as a first-line treatment of adult patients with metastatic NSCLC with activating epidermal growth factor receptor mutations.
Erbitux: Indicated both as monotherapy and in combination with another agent for the treatment of certain types of colorectal cancers; and as monotherapy, in combination with chemotherapy, or in combination with radiation therapy for the treatment of certain types of head and neck cancers.
Jaypirca: For the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) after at least two lines of systemic therapy, including a BTK inhibitor; and for the treatment of adult patients with chronic lymphocytic leukemia or small lymphocytic lymphoma who have received at least two prior lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor.
Retevmo: For the treatment of metastatic NSCLC with a rearranged during transfection (RET) gene fusion in adult patients; for the treatment of advanced metastatic medullary thyroid cancer with a RET mutation who require systemic therapy in adult and pediatric patients; for the treatment of advanced or metastatic thyroid cancer with a RET gene fusion in adult and pediatric patients who require systemic therapy and are radioactive iodine-refractory; and for the treatment of adult patients with locally advanced or metastatic solid tumors with a RET gene fusion who have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options.
Tyvyt: In collaboration with Innovent Biologics, Inc., for the treatment of relapsed or refractory classic Hodgkin's lymphoma; for the first-line treatment of non-squamous NSCLC in combination with Alimta and another agent; for the first-line treatment of squamous NSCLC in combination with two other agents; for the first-line treatment of hepatocellular carcinoma in combination with another agent; for the first-line treatment of esophageal squamous cell carcinoma in combination with certain other agents; for the first-line treatment of gastric cancer in combination with two other agents; and, in combination with two other agents, for patients with epidermal growth factor receptor (EGFR)-mutated non-squamous NSCLC that progressed after EGFR-tyrosine kinase inhibitor therapy, each in China.
Verzenio: For use as monotherapy or in combination with endocrine therapy for the treatment of HR+, HER2- metastatic breast cancer and in combination with endocrine therapy for the treatment of HR+, HER2-, node positive, early breast cancer at high risk of recurrence.
Immunology Products
Ebglyss: For the treatment of adult and adolescent patients 12 years or older with moderate to severe atopic dermatitis in Japan and, in collaboration with Almirall S.A., in Europe.
Olumiant: In collaboration with Incyte Corporation, for the treatment of adults with moderately to severely active rheumatoid arthritis after treatment with one or more tumor necrosis factor (TNF) blockers that did not work well enough or could not be tolerated; moderate to severe atopic dermatitis; severe alopecia areata; and for the treatment of hospitalized adults with COVID-19 who require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Omvoh: For the treatment of adults with moderately to severely active ulcerative colitis.
Taltz: For the treatment of adults and pediatric patients aged 6 years or older with moderate to severe plaque psoriasis; adults with active psoriatic arthritis; adults with ankylosing spondylitis; and adults with active non-radiographic axial spondyloarthritis.
Neuroscience Products
Cymbalta: For the treatment of major depressive disorder; diabetic peripheral neuropathic pain; generalized anxiety disorder; fibromyalgia; and chronic musculoskeletal pain due to chronic low back pain or chronic pain due to osteoarthritis.
Emgality: For migraine prevention and the treatment of episodic cluster headache in adults.
Other Products and Therapies
Cialis: For the treatment of erectile dysfunction and benign prostatic hyperplasia.
Forteo: For the treatment of osteoporosis in men and postmenopausal women at high risk for broken bones or fracture and for glucocorticoid-induced osteoporosis in men and women.
Marketing and Distribution
The company sells most of its products worldwide. The company adapts its marketing methods and product emphasis in various countries to meet local customer needs and comply with local regulations.
The U.S.
The company promotes its major products in the U.S. through sales representatives who engage with physicians and other healthcare professionals. The company also educates healthcare providers about its products in various other ways, including promoting in online channels, distributing literature and samples of certain products to physicians, and exhibiting at medical meetings. In addition, the company advertises certain products directly to consumers in the U.S., and it maintains websites and other media channels (e.g., social media) with information about its major products. The company supplements its employee sales force with contract sales organizations to leverage its resources and reach additional patients in need.
The company’s account managers service wholesalers, pharmacy benefit managers, managed care organizations, group purchasing organizations, government and long-term care institutions, hospitals, and certain retail pharmacies. The company enters into arrangements with these organizations to provide discounts or rebates on its products.
In the U.S., most of the company’s products are distributed through wholesalers that serve pharmacies, physicians and other healthcare professionals, and hospitals. In 2023, three wholesale distributors in the U.S.—McKesson Corporation, Cencora, Inc., and Cardinal Health, Inc.—each accounted for a significant percentage of the company’s consolidated revenue.
Outside the U.S.
The products the company markets and their distribution vary from country to country. Outside the U.S., the company promotes its products to healthcare providers through sales representatives and other channels. In most countries in which the company operates, it maintains its own sales organizations, but in some countries it markets its products through third parties, some of which it has engaged through distribution and promotion arrangements.
Marketing Collaborations
Certain of its products are marketed in arrangements with other pharmaceutical companies. For example, the company and Boehringer Ingelheim have a global agreement to develop and commercialize a portfolio of diabetes products, including Trajenta, Jentadueto, Jardiance, Glyxambi, Synjardy, Trijardy XR, Basaglar, and Rezvoglar.
Patents, Trademarks, and Other Intellectual Property Rights
The patent protection anticipated to be of most relevance to pharmaceuticals is provided by patents claiming the active ingredient (the compound patent) for the company’s products, particularly those in major markets, such as the U.S., major European countries, and Japan. In general, patents in each relevant country last for a period of 20 years from their filing date, which is often years prior to the launch of a commercial product. Further patent term adjustments and restorations may extend the original patent term:
Patent term adjustment is available to all U.S. patent applicants to provide relief in the event that a patent grant is delayed during examination by the U.S. Patent and Trademark Office (USPTO).
Patent term restoration for a single patent for a pharmaceutical product is provided to U.S. patent holders to compensate for a portion of the time invested in clinical trials and the U.S. Food and Drug Administration (FDA) review process. There is a five-year cap on any restoration, and no patent's expiration date may be extended beyond 14 years from FDA approval. Some countries outside the U.S. similarly offer forms of patent term restoration.
Outside the major markets, the adequacy and effectiveness of intellectual property protection for pharmaceuticals vary widely. International and U.S. free trade agreements like the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPs Agreement) administered by the World Trade Organization provide global protection of certain intellectual property rights. But in a number of markets the company is unable to patent its products or to enforce the patents that it receives for its products.
Intellectual Property Portfolio
The following product candidates are the most relevant that are under regulatory review. Upon approval, the company expects relevant compound patent and data protections to apply:
Donanemab has been submitted for regulatory review in the U.S., the EU and Japan for the treatment of early Alzheimer's disease.
Lebrikizumab has been submitted for regulatory review in the U.S. for the treatment of moderate to severe atopic dermatitis.
Pirtobrutinib has been submitted for regulatory review in Japan for the treatment of certain patients with relapsed or refractory mantle cell lymphoma.
Worldwide, the company sells all of its major products under trademarks consisting of its product names, logos, and unique product appearances that it considers in the aggregate to be important to its operations.
Government Regulation
Evolving regulatory priorities have intensified governmental scrutiny of the company’s operations and those of other healthcare intermediaries, including with respect to current Good Manufacturing Practices (cGMP), quality assurance, and similar regulations. Regulatory oversight of the pharmaceutical industry entails judgment and interpretation, which can result in inconsistent administration of laws and regulations by health authorities.
Of particular importance to its business is regulation by the FDA in the U.S. Pursuant to laws and regulations that include the Federal Food, Drug, and Cosmetic Act (FDCA), the FDA has jurisdiction over all of the company’s products and devices in the U.S. and administers requirements covering the testing, safety, effectiveness, manufacturing, quality control, distribution, labeling, marketing, promotion, advertising, dissemination of information, and post-marketing surveillance of those products and devices. The FDA holds broad discretion under the FDCA and other statutes to interpret the conditions and evidence necessary for timely approval of the company’s drugs and devices.
Outside the U.S., the company’s products and operations are subject to similar regulatory requirements, notably by the EMA in Europe, the Ministry of Health, Labor and Welfare in Japan, and the National Medical Products Administration in China. Specific regulatory requirements vary from country to country.
Regulators assess compliance with these regulations by inspecting the equipment, facilities, laboratories, and processes used in the manufacturing and testing of the company’s products prior to marketing approval with periodic reinspection thereafter; this may include inspection of its third-party business partners. For example, in 2023, the company received complete response letters based on FDA observations made during inspections of manufacturing facilities rather than any issues related to efficacy or safety. These resulted in certain delays in the approval of new products.
The company has implemented a Contract Pharmacy Limited Distribution System applicable to sales through the 340B program, which generally limits distribution of 340B-priced product to covered entities and their child sites; contract pharmacies wholly owned by the covered entity; or if a covered entity lacks an in-house outpatient pharmacy, a single contract pharmacy designated by a covered entity to establish a 340B bill to/ship to arrangement.
The U.S. Foreign Corrupt Practices Act of 1977 (FCPA) prohibits certain individuals and entities, including U.S. publicly traded companies, from promising, offering, or giving anything of value to foreign officials with the corrupt intent of influencing the foreign official for the purpose of helping the company obtain or retain business or gain any improper advantage. The FCPA also imposes specific recordkeeping and internal controls requirements on the U.S. publicly traded companies. As noted above, the company’s business is heavily regulated and therefore involves significant interaction with officials outside the U.S.
History
Eli Lilly and Company was founded in 1876 by Colonel Eli Lilly. The company was incorporated in 1901 in Indiana.

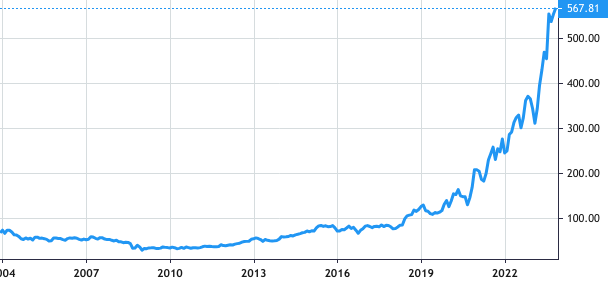
 Stock Value
Stock Value

