About Boston Scientific
Boston Scientific Corporation operates as a global developer, manufacturer, and marketer of medical devices that are used in a broad range of interventional medical specialties.
As a medical technology leader, the company has advanced the practice of less-invasive medicine by helping physicians and other medical professionals diagnose and treat a wide range of diseases and medical conditions and improve patients’ quality of life by providing alternatives to surgery and other medical procedures that are typically traumatic to the body. The company advances science for life by providing a broad range of high performance solutions to address unmet patient needs.
Business Strategy
The company operates pursuant to five strategic imperatives, such as strengthening category leadership, expanding into high growth adjacencies, driving global expansion, funding the journey to fuel growth, and developing key capabilities.
Maintaining and expanding the company’s international presence is an important component of the company’s long-term growth strategy. The company’s research and development efforts are focused largely on the development of next-generation and novel technology offerings across multiple programs and all divisions.
The company continues to develop digital tools and technologies that enable the company to compete more effectively and deliver first class remote physician education, drive deeper patient engagement and increase digitally-enabled sales force productivity.

Product Offerings
The company’s core businesses are organized into two reportable segments: MedSurg and Cardiovascular. The following describes the company’s key product offerings and new product innovations by reportable segment and business unit.
MedSurg
Endoscopy
The company’s Endoscopy business develops and manufactures devices to diagnose and treat a broad range of gastrointestinal (GI) and pulmonary conditions with innovative, less invasive technologies. The company’s product offerings include the following:
Resolution 360 Clips and Resolution 360 ULTRA Clips, hemostatic clipping technology designed to stop and help prevent bleeding during endoscopic procedures;
WallFlex Biliary Stent Systems, used for relieving biliary obstructions by providing bile drainage in both malignant and benign strictures;
AXIOS Stents and Electrocautery Enhanced Delivery Systems, the first, and only stent systems in the U.S. indicated for endoscopic drainage of pancreatic pseudocysts and used to facilitate endoscopic drainage of the gallbladder for patients with acute cholecystitis;
SpyGlass DS II Direct Visualization Systems and SpyGlass Discover Digital Catheters, the first single-use scopes to enable physicians to take a single-stage approach to diagnostic and therapeutic procedures in the pancreaticobiliary system, including treating patients with bile duct stones;
EXALT Model D Single-Use Duodenoscopes for use in endoscopic retrograde cholangiopancreatography (ERCP) procedures, the first U.S. Food and Drug Administration (FDA)-cleared single-use (disposable) duodenoscopes on the market; and
The company’s infection prevention portfolio, designed to minimize the risk of infection transmission and improve operational efficiencies by streamlining manual cleaning or eliminating the need for cleaning and tracking.
In the second quarter of 2023, the company completed the acquisition of Apollo Endosurgery, Inc., a public company that has developed and commercialized endoscopic suturing devices, including OverStitch Endoscopic Suturing Systems and X-Tack Endoscopic HeliX Tacking Systems and endobariatric devices, including the Apollo ESG and Apollo REVISE Systems, the first devices authorized by the FDA for endoscopic sleeve gastroplasty and endoscopic bariatric revision procedures, as well as the Orbera Intragastric Balloon for endoscopic weight management.
Urology
The company’s Urology business develops and manufactures devices to treat various urological conditions for both male and female anatomies, including kidney stones, benign prostatic hyperplasia (BPH), prostate cancer, erectile dysfunction, and incontinence. The company’s product offerings include the following:
A comprehensive line of stone management products, including ureteral stents, catheters, baskets, guidewires, sheaths and balloons;
LithoVue Single-Use Digital Flexible Ureteroscopes, which deliver detailed high-resolution digital images for high-quality visualization and seamless navigation;
Lumenis Pulse Holmium Laser Systems with MOSES Technology, complemented by a full line of laser fibers and accessories used in urology procedures;
The company’s prosthetic urology portfolio, which includes the company’s AMS 700 penile implant to treat erectile dysfunction and the company’s AMS 800 Artificial Urinary Sphincter to treat male urinary incontinence,
GreenLight XPS Laser System, MoXy Fiber, and Rezum Systems for the treatment of BPH; and
SpaceOAR Hydrogel Systems which help reduce side effects that men may experience after receiving radiotherapy to treat prostate cancer, together with the company’s SpaceOAR VUE Hydrogel, providing clinicians with enhanced product visualization.
In the first quarter of 2023, the company received FDA clearance for and launched its LithoVue Elite Single-Use Digital Flexible Ureteroscope System, the first ureteroscope with the ability to monitor intrarenal pressure in real-time during ureteroscopy procedures.
Pending Axonics Acquisition
On January 8, 2024, the company announced its entry into a definitive agreement to acquire Axonics, Inc. (Axonics), a publicly traded medical technology company primarily focused on the development and commercialization of devices to treat urinary and bowel dysfunction. The Axonics product portfolio includes the Axonics R20 and Axonics F15 Systems used to deliver sacral neuromodulation (SNM) therapy for the treatment of over-active bladder and fecal incontinence. The transaction is expected to close in the first half of 2024, subject to customary closing conditions. The company plans to fund the acquisition through a mix of cash on hand and new debt. The Axonics business will be integrated into the company’s Urology division.
Neuromodulation
The company’s Neuromodulation business develops and manufactures devices to treat various neurological movement disorders and manage chronic pain. The company’s product offerings include the following:
Precision Montage and WaveWriter Alpha Spinal Cord Stimulator (SCS) Systems, designed to provide improved pain relief to a wide range of patients who suffer from chronic pain, with proprietary features, such as Multiple Independent Current Control, the company’s Illumina 3D Proprietary Programming Software and FAST Therapy for profound parathesia-free pain relief in minutes, used by physicians to target specific areas of pain and customize stimulation of nerve fibers more precisely;
The company’s G4 Generator and consumable portfolio in Radiofrequency Ablation (RFA) for pain management used by physicians to treat patients with chronic pain;
Superion Indirect Decompression Systems, minimally-invasive devices used to improve physical function and reduce pain in patients with moderate lumbar spinal stenosis (LSS);
The company’s Cognita Practice Optimization suite of tools designed to increase awareness, streamline patient management, and sustain long-term outcomes for patients; and
Vercise Genus Deep Brain Stimulation (DBS) System for the treatment of Parkinson's disease, tremor, and intractable primary and secondary dystonia, a neurological movement disorder characterized by involuntary muscle contractions, utilizing Stimview XT, the company’s proprietary DBS visualization software developed in collaboration with Brainlab AG, providing clinicians with real-time, 3D visualization and stimulation of brain anatomy.
In the second quarter of 2023, the company received FDA approval for the Vercise Neural Navigator 5 Software, which when used with the Vercise Genus DBS systems can help provide clinicians with simple and actionable data for efficient programming in the treatment of people living with Parkinson's disease or essential tremor.
In addition, in the fourth quarter of 2023, the company completed the acquisition of Relievant Medsystems, Inc., a privately held medical technology company that has developed and commercialized the Intracept Intraosseous Nerve Ablation System, the only FDA-cleared system to treat vertebrogenic pain, a form of chronic low back pain.
Cardiovascular
Cardiology
Interventional Cardiology Therapies (ICTx)
The company’s Interventional Cardiology Therapies business develops and manufactures technologies for diagnosing and treating coronary artery disease and aortic valve conditions. The company’s product offerings include the following:
OptiCross Intravascular Ultrasound (IVUS) Imaging Catheters;
iLab Ultrasound Imaging Systems with Polaris Software, designed to enhance the diagnosis and treatment of blocked vessels and other heart disorders, compatible with the company’s full line of imaging catheters;
AVVIGO Guidance Systems and AVVIGO Guidance System II, incorporating high-definition IVUS all in a mobile or integrated platform;
ROTAPRO Rotational Atherectomy Systems, designed to treat coronary calcification in lesions by regulating the flow of air to the advancer, controlling burr rotation speed, and also monitoring and displaying burr rotation speed and rotational atherectomy procedural time;
SYNERGY, SYNERGY MEGATRON and SYNERGY XD Everolimus-Eluting Platinum Chromium Coronary Stent Systems, featuring an ultra-thin abluminal (outer) bioabsorbable polymer coating;
Safari2 Pre-Shaped Guidewires, intended to facilitate the introduction and placement of interventional devices within the heart;
WOLVERINE Coronary Cutting Balloon, a cutting balloon angioplasty device with a unique mechanism of action that enables precise vessel preparation across a wide range of resistant lesions;
AGENT Drug-Coated Balloon, which is designed to provide a targeted, therapeutic dose of anti-proliferative paclitaxel to the coronary lesion and minimize downstream particulates;
ACURATE neo2 Aortic Valve Systems for use in transcatheter aortic valve replacement (TAVR) procedures; and
SENTINEL Cerebral Embolic Protection Systems, used to reduce the risk of stroke in TAVR procedures and is clinically proven to decrease cerebral embolization and its associated neurological effects.
In the third quarter of 2023, the company received CE Mark, FDA clearance and Japanese Pharmaceuticals and Medical Devices Agency (PMDA) approval for the AVVIGO+ Multi-Modality Guidance System, a next-generation technology that provides high-quality IVUS imaging and physiologic assessment of coronary vessels and lesions.
Watchman
The company’s WATCHMAN FLX Left Atrial Appendage Closure (LAAC) Devices are designed to close the left atrial appendage in patients with non-valvular atrial fibrillation who are at risk for ischemic stroke. WATCHMAN is the first device to offer a non-pharmacologic alternative to oral anti-coagulants that has been studied in a randomized clinical trial and is the leading device in percutaneous LAAC globally. In the third quarter of 2023, the company received FDA approval for the latest-generation WATCHMAN FLX Pro LAAC Device, which is designed to improve visualization during device placement, reduce device-related thrombus post-implant and treat a broader range of patient anatomies.
Cardiac Rhythm Management
The company’s Cardiac Rhythm Management (CRM) business develops and manufactures a variety of implantable devices that monitor the heart and deliver electricity to treat cardiac abnormalities. The company’s product offerings include the following:
The RESONATE family of implantable cardioverter defibrillators (ICD) and implantable cardiac resynchronization therapy defibrillators (CRT-D), including the company’s proprietary HeartLogic Heart Failure (HF) Diagnostic and SmartCRT Technology with Multisite pacing in CRT-D;
EMBLEM MRI S-ICD Systems, the world's first commercially available subcutaneous implantable cardiac defibrillators (S-ICD), which provides physicians the ability to treat patients who are at risk for sudden cardiac arrest without touching the heart;
ACCOLADE family of pacemakers and implantable cardiac resynchronization therapy pacemakers (CRT-P);
ACUITY X4 Quadripolar LV Leads, RELIANCE family of ICD Leads and the company’s INGEVITY Pacing Leads;
LATITUDE Remote Patient Management Systems, which allow for more frequent monitoring and better guided treatment decisions by enabling physicians to monitor implantable system performance remotely;
LUX-Dx Insertable Cardiac Monitor (ICM) systems, long-term diagnostic devices implanted in patients to detect arrhythmias associated with conditions, such as atrial fibrillation (AF), cryptogenic stroke and syncope; and
BodyGuardian Remote Cardiac Monitoring Systems provide a full range of mobile health solutions and remote monitoring services, ranging from ambulatory cardiac monitors – including short and long-term holter monitors – to cardiac event monitors and mobile cardiac telemetry.
In the third quarter of 2023, the company received FDA clearance and launched the next-generation LUX-Dx II/II+ ICM system for long-term monitoring of arrhythmias. Additionally, the company’s entire transvenous defibrillator portfolio leverages its EnduraLife Battery Technology and has magnetic resonance imaging (MRI) conditional labeling when used with the company’s generation of leads.
Electrophysiology
The company’s Electrophysiology business develops and manufactures less-invasive medical technologies used in the diagnosis and treatment of rate and rhythm disorders of the heart, including a broad portfolio of therapeutic and diagnostic catheters and a variety of equipment used in the Electrophysiology lab. The company’s product offerings include the following:
Farapulse Pulsed Field Ablation (PFA) System for the treatment of AF;
POLARx Cryoablation Systems for the treatment of AF;
VersaCross Connect Access Solutions for the company’s WATCHMAN FXD Curve Sheath, Polarsheath and Faradrive Steerable Sheath providing safe and efficient access to the left side of the heart;
Rhythmia Mapping Systems, catheter-based, 3-D cardiac mapping and navigation solutions designed to help diagnose and guide treatment of a variety of arrhythmias;
A portfolio of radiofrequency (RF) cardiac ablation catheters, including the company’s INTELLANAV STABLEPOINT catheter, which also includes DIRECTSENSE Software for monitoring RF energy during ablations; and
IntellaMap Orion Mapping Catheters, for use with the company’s Rhythmia Mapping System to provide high-density, high-resolution maps of the heart.
In the third quarter of 2023, the company received FDA approval for the POLARx Cryoablation System, which includes the POLARx FIT Cryoablation Balloon Catheter, and in the first quarter of 2024, the company received FDA approval for the FARAPULSE PFA System, both of which are used to treat patients with paroxysmal AF.
Peripheral Interventions
The company’s Peripheral Interventions business develops and manufactures products to diagnose and treat peripheral arterial and venous diseases, as well as products to diagnose, treat and ease various forms of cancer. The company’s broad peripheral portfolio includes stent systems, balloon catheters, guidewires, atherectomy and thrombectomy systems, embolization devices, radioactive microspheres, radiofrequency and cryotherapy ablation systems, microcatheters and drainage catheters.
The company’s peripheral arterial product offerings include:
Eluvia Drug Eluting Vascular Stent Systems, innovative stents built on the Innova stent platform, designed to deliver a sustained dosage of paclitaxel during the time when restenosis is most likely to occur, in addition to the Eluvia line extension, the longest-length stent available for the treatment of patients with peripheral artery disease (PAD) in the superficial femoral artery;
Mustang, Coyote and Sterling PTA Balloon Catheters designed for a wide variety of peripheral angioplasty procedures; and
Ranger Drug-Coated Balloons, innovative balloons built on the Sterling balloon platform, featuring a low-dose of paclitaxel.
The company’s venous disease product offerings include the following:
AngioJet Thrombectomy Systems, used in endovascular procedures to remove blood clots from blocked arteries and veins and the company’s AngioJet Zelante DVT Thrombectomy Catheters to treat deep vein thrombosis;
EKOS Ultrasound Assisted Thrombolysis systems used to treat pulmonary embolisms; and
Varithena Polidocanol Injectable Foam used to improve the symptoms of superficial venous incompetence and the appearance of visible varicosities.
The company’s interventional oncology product offerings include the following:
TheraSphere Y-90 radioactive glass microspheres used in the treatment of hepatocellular carcinoma (HCC), the most common type of liver cancer;
Renegade HI-FLO Fathom Microcatheter and Guidewire System and Interlock - 35 Fibered IDC and 18 Fibered IDC Occlusion System for peripheral embolization;
EMBOLD Detachable Coil System, used for arterial and venous embolizations in the peripheral vasculature; and
ICEFX and Visual ICE Cryoablation Systems for destruction of tissue, using image-guided needles to enable cryoablation visualization for optimal tumor coverage.
In the first quarter of 2023, the company acquired a majority stake investment in Acotec Scientific Holdings Limited (Acotec), a publicly traded Chinese manufacturer of drug-coated balloons and other products used in the treatment of vascular and other diseases, complementing the company’s existing Peripheral Interventions portfolio. In addition, in the second quarter of 2023, the company received FDA 510(k) clearance for the EMBOLD Soft and Packing Coils, which, along with the EMBOLD Fibered Coil, complete the EMBOLD Detachable Coil System, a peripheral embolization platform for vessel occlusion designed to simplify operator workflow and streamline inventory for hospitals. In the fourth quarter of 2023, the company started to introduce the company’s OBSIDIO Conformable Embolic for use in the embolization of hypervascular tumors and blood vessels to occlude blood flow for controlling bleeding/hemorrhaging in the peripheral vasculature.
Competition
The company’s primary competitors include Abbott Laboratories and Medtronic plc.
Marketing and Sales
The company markets its products and solutions to hospitals, clinics, outpatient facilities and medical offices in 140 countries worldwide. In addition, large group purchasing organizations, hospital networks and other buying groups are important to the company’s business and represent a substantial portion of the company’s net sales. Each of the company’s businesses maintains dedicated sales forces and marketing teams focused on physicians who specialize in the diagnosis and treatment of different medical conditions, as well as on key hospital service line administrators.
The majority of the company’s net sales are derived from countries in which the company has direct sales organizations. The company also has a network of distributors and dealers who offer the company’s products in certain countries and markets. The company expects to continue to leverage its infrastructure in markets where commercially appropriate and use third party distributors in those markets where it is not economical or strategic to establish or maintain a direct presence.
Regulatory Environment
The medical devices that the company manufactures, markets and commercializes are subject to regulation by numerous worldwide regulatory bodies, including the FDA and comparable international regulatory agencies. These agencies require manufacturers of medical devices to comply with applicable laws and regulations governing development, testing, manufacturing, labeling, marketing and distribution.
In the European Union (EU), the company is required to comply with the Medical Device Regulation (MDR or EU MDR), which became effective in May 2021, superseding the existing Medical Device and Active Implantable Medical Device Directives. Medical devices, which have a valid CE Certificate to the prior Directives (issued before May 2021) can continue to be sold during the applicable transition period or until the CE Certificate expires, whichever comes first, providing there are no significant changes to the design or intended use.
In Japan, the company is required to comply with Japan’s Ministry of Health, Labor and Welfare (MHLW) regulations.
The company’s quality system is designed to enable the company to satisfy various international quality system regulations, including those of the FDA with respect to products sold in the U.S. The International Standards Organization (ISO) established the ISO 13485 quality system standard, which includes requirements for an implemented quality system that applies to component quality, supplier control, product design and manufacturing operations. All of the company’s medical device manufacturing facilities and key distribution sites are certified under the ISO 13485 quality system standard.
The company’s products are purchased principally by hospitals, physicians and other health care providers around the world that typically bill various third-party payers, including government programs (e.g., Medicare and Medicaid in the U.S.) and private insurance payers, for the items and services provided to their patients.
Seasonality
While the company’s consolidated net sales do not reflect any significant degree of seasonality, customer purchases of the company’s medical devices have historically been lower in the first and third quarters of the year (year ended December 2023).
History
Boston Scientific Corporation was founded in 1979. The company was incorporated in 1979.

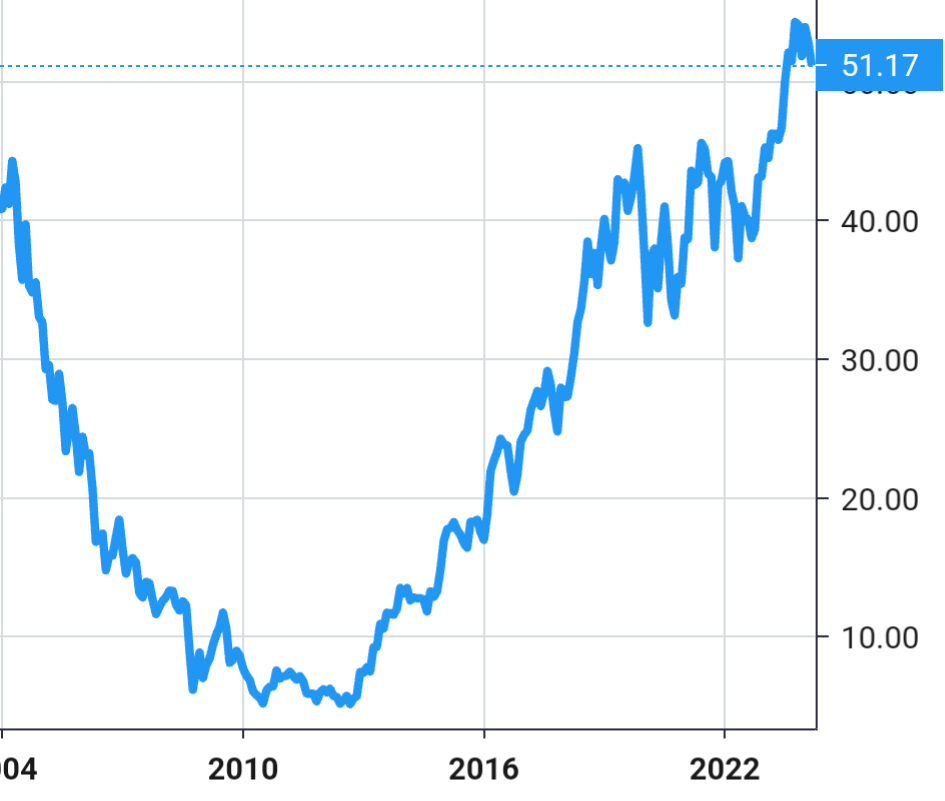
 Stock Value
Stock Value

